International Journal of
eISSN: 2574-9889


Research Article Volume 4 Issue 4
1Laboratory of Physiology, Pharmacology and Pharmacopoeia, Nangui Abrogoua University, Cote d'Ivoire
2Biological Sciences Training and Research Unit, Peleforo Gon Coulibaly University, Cote d'Ivoire
Correspondence: Bleyere N Mathieu, Laboratory of Physiology, Pharmacology and Pharmacopoeia, Nangui Abrogoua University, 02 BP 801 Abidjan 02, Cote d'Ivoire, Tel 22545439944
Received: August 08, 2018 | Published: August 22, 2018
Citation: Aristide AJP, Soualio K, MathieuBN, et al. Assessment of some nutritional blood parameters during pregnancy at southern Abobo hospital (Abidjan, Côte d’Ivoire). Int J Pregn & Chi Birth. 2018;4(4):209-216. DOI: 10.15406/ipcb.2018.04.00111
During pregnancy, physiological adjustments that may result in increased demand for energy and maternal food occur to support fetal development. In order to evaluate the effect of these metabolic variations on nutritional blood biomarkers, this study was undertaken on pregnant women at different stages of pregnancy. Thus, 150 pregnant women were recruited, by reason of 50 women per trimester of pregnancy, based on our inclusion and non-inclusion criteria. The average age of women was 26.4±0.5 years ranging from 18 to 35 years of age. From each fasted woman, blood was taken at the crease of the elbow in the morning. This blood was collected in tubes containing anticoagulant EDTA, anticoagulant sodium fluoride + potassium oxalate and tubes without anticoagulant for hematological and biochemical parameters determination. Hematological findings showed four types of anemia (hypochromic normocytic anemia, hypochromic microcytic anemia, normochromic normocytic anemia and normochromic microcytic anemia). Hypochromic microcytic anemia was highest (20%), in women being in their first trimester of pregnancy. Then, the latter type of anemia and hypochromic normocytic anemia (16%) simultaneously shared this preponderance of anemia, in the group of women being in their second trimester of pregnancy. Finally, among women in the third trimester of pregnancy, the prevalence of normochromic normocytic anemia (30%) was highest. At the biochemical level, a non- significant increase in HDL hypercholesterolemia, as well as a non- significant decrease in LDL cholesterol and sodium were noted. In addition, conjugated bilirubin was decreased significantly in the third trimester. In contrast, significant growth in hyperkalemia was noted in the third trimester. In addition, significant decreasing mean of conjugated bilirubin and increasing potassium were noted. Finally, a very highly significant increase in chlorine in the third term was recorded. The study revealed different types of more acute anemias in women being in their last term of pregnancy, and variations in serum potassium, chloride and bilirubin.
Keywords: pregnancy, blood parameters, nutritional markers, anemia
Pregnancy is a dynamic and anabolic state. It consists of a series of continuous physiological adjustments with the formation of a new endocrine organ which is the placenta; it secretes hormones that can affect the metabolism of all foods.1 These adjustments in nutrient metabolism, in addition to changes in the anatomy and physiology of the mother, support growth and fetal development while maintaining maternal homeostasis. These adjustments can vary considerably from one woman to another according to the diet.1 During this period, the demand for energy, maternal and fetal food is increased. For well-nourished women, only little additional energy is required, because the body adapts to increased energy needs.2,3 Indeed, it is known that nutrition and maternal health have an impact on the well-being of the foetus and predict the health of the child.4 Thus, nutritional status during pregnancy can partially influence outcome of pregnancy and birth.5 It is therefore important to detect early nutritional dysfunction, so that women food consumption can be improved. To do this, it is possible to evaluate some substances present in tissues and fluids. These substances, called biomarkers, are considered bio-indicators. These are biological specimens, objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, markers of exposure to a substance and its metabolism.6,7 Biomarkers may also reflect the characteristics of the host. They are used in a broad sense to include almost any measure that reflects an interaction between a biological system and a chemical, physical or biological product of the environment.6 Dietary deficiency during pregnancy can cause delay in fetal development, premature delivery, low birth weight, fetal distress and maternal deaths during pregnancy, during or after delivery8–11 Pregnancy is therefore associated with a significant medical, nutritional, social and economic risk for mothers and their infants. Despite this, relatively little is known about the nutritional status of populations.12 Studies on the nutritional status of the mother are rare and cannot be transposed from one country to another. In Côte d'Ivoire, the nutritional status of pregnant women has been the subject of only a few studies dealing with nutritional status in relation to micronutrient stores, particularly metabolic iron.13–19 The objective of this study is to measure and evaluate some blood nutritional biomarkers of pregnant women attending Abobo Hospital in order to appreciate their nutritional status in different stages of pregnancy.
Population
A cross-sectional study that ran from November 2016 to March 2017 at the Abobo south General Hospital. The study involved 150 our survey took into account 150 pregnant women at the rate of 50 per term aged between 18 and 35 years. . The inclusion of these women was based on documents provided by health workers and survey cards. Thus, pregnant women with no diseases such as diabetes, high blood pressure, rheumatism, HIV, hepatitis and who have not recently been transfused with blood have been included. The average age of the subjects in our study is 26.4±0.5 years. The average number of pregnancies carried by women in our study is 2.37±0.1. The parity average is 1.24±0.1 and the average number of months between the last parity and the current gestational age is 28.24±2.8. During the three trimesters of pregnancy, body mass index (BMI) values increased from 24.47±0.7 kg.m-2 to 27.11±0.8 kg.m-2 and 26.33±0.6 kg.m-2 respectively in the first, second and third trimesters of pregnancy. In Table 1, we note that the body mass index is abnormal (insufficient or highter than normal) in 44.7% of cases. In addition, there was an increase in BMI variation per trimester from 0.43 to 1.85 and then to 3.53, respectively, in the first, second and third trimesters of pregnancy. Multiskilled (66%), nulliparous (39%), no-schooled (46%), commercial (48%) and concubine (79.3%) women had high proportions in our study.
Anthropometric and sociodemographic |
Total population |
1rsttrimester |
2ndtrimester |
3rdtrimester |
|---|---|---|---|---|
Age (years) |
26.40±0.5 |
26.24±0.8 |
27.32±0.8 |
25.64±0.8 |
BMI (Kg.m-2) |
25.97±0.4 |
24.47±0.7 |
27.11±0.8 |
26.33±0.6 |
Gravidity |
2.37±0,1 |
1.88±0.1 |
2.68±0,2 |
2.56±0.2 |
Parity |
1.24±0,1 |
0.78±0.2 |
1.52±0.2 |
1.42±0.2 |
Time between births (months) |
28.24±2,8 |
27.6±6.03 |
29.04±4.06 |
28.08±4.3 |
Educational attainment |
69 (46 %) |
19 (38 %) |
23 (46 %) |
27 (54 %) |
Matrimonial status |
7 (4.7 %) |
2 (4 %) |
3 (6 %) |
2 (4 %) |
Profession |
72 (48 %) |
20 (40 %) |
27 (54 %) |
25 (50 %) |
Number of meals per day |
29 (19.3 %) |
9 (18 %) |
12 (24 %) |
8 (16 %) |
Antianemic |
96 (64 %) |
29 (48 %) |
29 (48 %) |
38 (76 %) |
Table 1 General characteristics of the study population
N, Total number of subject group; n, Number of subject observed in each group; BMI, Body mass index
Blood samples and assays of blood nutritional biomarkers
From each of the recruited women blood was collected under aseptic conditions into sterile 5mL blood tubes containing EDTA anticoagulant, sodium fluoride + potassium oxalate and no anticoagulant. Pregnant women who come for consultation are removed on an empty stomach in the morning, by venipuncture at the elbow, depending on the trimester of pregnancy. The hematological parameters consist of variables related to the blood count (NFS). A few minutes after collection, the hematological parameters were determined using tubes containing EDTA anticoagulant, using a Rayto RT 7600S automated hematology analyzer (Shenzen, China). Blood samples in the dry tubes and gray tubes are centrifuged at 1107 Newton for three (3) minutes to obtain the serum. This serum was used for biochemical assay of nutritional biomarkers using the Prietest Touch Robonik semi-automated machine (Mahape, Navi Mumbai, India). Blood glucose was also determined a few minutes after centrifugation. Blood glucose, serum creatinine, total protein, calcium, chlorine, total and direct bilirubin were determined using a colorimetric method with their respective reagents. Total cholesterol, HDL cholesterol and triglycerides were estimated by an enzymatic method and LDL cholesterol was determined by a calculation method. Potassium estimation is done by turbidimetric method. The protocol and experimental procedures used in this study were approved by the Ministry of Higher Education and Scientific Research of Côte d'Ivoire, the authorities of the Nangui Abrogoua University and the Department of Establishments and Professions Sanitary facilities (DEPS).
Statistical analyzes of blood biological parameters
The statistical analysis of the data was carried out with the computer program (Graphpad prism version 5.01). The results were given as average followed by the standard error on the mean (M±SEM). The comparison of the means of the biological markers between the different trimesters of the pregnancy was carried out by analysis of variances (ANOVA) for the independent samples. This analysis aims to highlight a probable variation of nutritional biomarkers according to the different stages of pregnancy. All the analyzes of variances carried out were associated to the Tukey multiple comparison post hoc test, in order to specify a possible significant difference between women. The different observed proportions of biological parameters among women's groups were compared by the G test or test log likehood ratio with the Windows version R.2.0.1 software.20
Distribution of hematological parameters of pregnant women
The results of the study reported in Table 2 showed that mean values of erythrocyte parameters in pregnant women in Abobo area have been deteriorated. They were lower than normal for hemoglobin levels, mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). On the other hand, the comparison of the means of the hematological parameters according to the different trimesters of the pregnancy, showed a highly significant decrease (p=0.003) at the level of the red blood cells, of the hemoglobin (p=0,0005) and hematocrit (p=0.003). In addition, the average of the parameters of the blood count decreased gradually throughout the pregnancy. On the erythrocyte indices, including MCV, MCH and MCHC, the comparison of averages of these parameters per trimester, showed no change during pregnancy. Leukocyte and thrombocyte parameters were increased but still consistent with reference values during the three trimesters of pregnancy. Also, comparison of the averages of these parameters by trimester did not reveal any significant difference.
Hematological |
Stages of pregnancy (trimester) |
Reference values |
P-value |
||
|---|---|---|---|---|---|
First |
Second |
Third |
|||
Blood count |
|
|
|
|
|
Redcells(106/mm3) |
4.34±0.11 |
3.95±0.07 |
4.01±0.07 |
4 – 5.4 |
0.003a |
Hemoglobin (g/dl) |
11.36±0.18 |
10.55±0.16 |
10.56±0.14 |
11 – 16 |
0.0005b |
Hematocrit (%) |
34.47±0.53 |
32.55±0.44 |
32.41±0.45 |
32 – 47 |
0.003c |
Erythrocyte indices |
|
|
|
|
|
MCV (fl) |
81.86±0.92 |
82.79±0.91 |
81.92±0.84 |
80 – 100 |
0.715 |
MCH (pg) |
25.42±0.57 |
25.25±0.59 |
26.12±0.43 |
27 – 31 |
0.476 |
MCHC (g/dl) |
31.13±0.52 |
30.27±0.48 |
31.54±0.35 |
32 – 36 |
0.137 |
Leukocyte and thrombocyte parameters |
|
|
|
||
Leukocytes(103/mm3) |
6.25±0.29 |
6.71±0.29 |
6.69±0.26 |
4 – 10 |
0.430 |
Neutrophils (103/mm3) |
3.81±0.22 |
4.23±0.18 |
4.21±0.19 |
1.7 – 7 |
0.248 |
Eosinophils (103/mm3) |
0.08±0.01 |
0.09±0.01 |
0.09±0.01 |
0 – 0.5 |
0.652 |
Lymphocytes (103/mm3) |
1.86±0.09 |
1.85±0.15 |
1.82±0.08 |
1.5 – 4 |
0.963 |
Monocytes (103/mm3) |
0.49±0.03 |
0.53±0.03 |
0.56±0.03 |
0.1 – 1 |
0.165 |
Thrombocytes (103/mm3) |
221.6±9.0 |
228±10.8 |
232.2±8 |
150 – 400 |
0.720 |
Table 2 Hematological parameters during pregnancy
MCV, Mean corpuscular volume; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; a, b, c, Statistically different value for p <0.05.
Variations in the biochemical parameters of pregnant women
Table 3 shows the evolution of biochemical parameters at different stages of pregnancy. It reveals that the average value of HDL-cholesterol has exceeded the reference values. On the other hand, the average values of LDL cholesterol, sodium and chlorine were below normal values. Excepted potassium, which level was above normal in the second and third trimesters of pregnancy, all other biochemical parameters remained normal. These results indicated also significant decreases in conjugated bilirubin (p=0.011) and potassium (p=0.025) on the one hand, and very highly significant (p=0.0001) on chlorine. In addition, the average values of triglyceride, total cholesterol, HDL and potassium levels were high, compared to reference values, at all stages of pregnancy.
Biochemical |
Stage of pregnancy (trimester) |
Reference |
p-value |
||
|---|---|---|---|---|---|
First |
Second |
Third |
|||
Glycemia (g/l) |
0.77±0.02 |
0.81±0.02 |
0.78±0.02 |
0.6 – 1.1 |
0.228 |
Creatinine (mg/l) |
7.42±0.25 |
6.98±0.21 |
7.21±0.2 |
6 – 17 |
0.348 |
Triglycerides (mg/l) |
0.87±0.07 |
0.97±0.06 |
1.06± 0.07 |
0.4 – 1.4 |
0.128 |
Totalcholesterol (g/l) |
1.57±0.07 |
1.74±0.09 |
1.76±0.08 |
1.5 – 2.32 |
0.185 |
HDL cholestérol (g/l) |
0.82±0.07 |
0.89±0.09 |
0.94±0.09 |
0.4 – 0.75 |
0.436 |
LDL cholestérol (g/l) |
0.75±0.07 |
0.80±0.09 |
0.68±0.08 |
1.08–1.88 |
0.588 |
ASAT (UI/l) |
21.57±1.2 |
21.21±1.1 |
20.51±1.2 |
7 – 37 |
0.808 |
ALAT (UI/l) |
15.7±0.79 |
17.05±1.15 |
15.76±1.1 |
6 – 40 |
0.575 |
Total protein(g/l) |
65.93±2.7 |
64.51±2.33 |
66.77±2.4 |
66 – 83 |
0.811 |
Total bilirubin (mg/l) |
4.66±0.58 |
4.88±0.52 |
4.17±0.65 |
3 – 10 |
0.681 |
Conjugated bilirubin (mg/l) |
2.43±0.34 |
1.7±0.22 |
1.36±0.17 |
< 4 |
0.011a |
Calcium (mg/l) |
100.3±2.3 |
96.34±3.16 |
98,96±2.8 |
81 – 104 |
0.589 |
Sodium (meq/l) |
125.3±3.1 |
121.97±3.9 |
130.8±3.1 |
135 – 155 |
0.181 |
Potassium (meq/l) |
4.76±0.18 |
5.35±0.2 |
5.42±0.18 |
3.5 – 5 |
0.025b |
Chloride (meq/l) |
86.38±2.3 |
82.53±1.54 |
98.24±3.7 |
90 – 105 |
0.0001c |
Table 3 Distribution of biochemical parameters by stage of pregnancy
ASAT, Aspartate aminotransferase; ALAT, Alanine aminotransferase; LDL, Low density lipoprotein; HDL, High density lipoprotein; a,b,c, Statistically different value for p < 0.05.
Prevalence of different types of anemia at stages of pregnancy
Figures 1 (A & B) presents the comparison of the proportions of the different types of anemia according to the stages of pregnancy. According to the results presented in Figure 1A, the prevalence of anemia is higher among women in the third trimester (56%) followed by those of the second trimester (44%) than women in their first trimester of pregnancy (36%). On the other hand, the comparison of the three proportions of anemia between them showed no significant difference (p>0.05). Concerning the variation of the erythrocyte parameters, hypochromic normocytic anemia (HNA), the hypochromic microcytic anemia (HmA), normochromic normocytic anemia (NNA) and normochromic microcytic anemia (NmA) were the different types of anemia observed in pregnant women of our study (Fig. 1B). But, a disappearance of the ANm is observed, in the women being in second and third trimester of the pregnancy. While, ANN has experienced a growing evolution according to the stages of pregnancy. In addition the comparison of the same type of anemia among the three groups of women (first trimester, second trimester and third trimester) showed a significant increase (p=0.03) of the NNA in the third trimester, compared to the other two groups trimesters. In women on their first trimester of pregnancy, the prevalence of HmA was the highest with 20%. In contrast, among those in the second trimester, the HmA and the HNA were simultaneously the highest with a prevalence of 16%. The predominant type of anemia, in women in their third trimester of pregnancy was the NNA, with a prevalence of 30%. In addition, the comparison of the prevalence of types of anemia according to the stages of pregnancy is carried out. It showed that women on their first trimester of pregnancy a significant statistical difference (p=0.01) of the HmA between the three other types of anemias. Then, a significant difference (p=0.048) of the NNA, compared to the other two types of anemia observed in women being in the third trimester is noted. Finally, there is a disappearance of NmA in groups of women in the second and third trimesters of pregnancy.
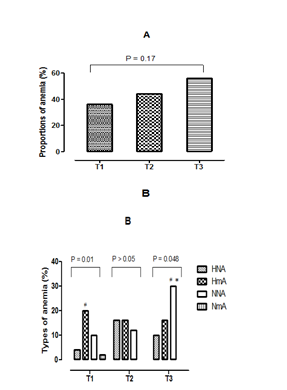
Figure 1 (A&B) Proportions of anemia and different types of anemia.
T1, First trimester of pregnancy; T2, Second trimester of pregnancy; T3, Third trimester of pregnancy; A, Proportions of anemia; B, Proportions of different types of anemia, HNA, Hypochromic normocytic anemia; HmA, hypochromic microcytic anemia; NNA, normochromic normocytic anemia, NmA, normochromic microcytic anemia; *, Statistical difference (p <0.05) between trimesters for the same type of anemia; #, Statistical difference (p <0.05) between the types of anemia observed in the same trimester.
Proportions of some hematological and biochemical parameters according to the stages of the pregnancy
Table 4 shows the proportions of some hematological and biochemical parameters according to the stages of pregnancy. In terms of hematological parameters, hemodilution was higher for women in the third trimester (50%) than for women in the second (42%) and third trimester (36%). Microcytosis was higher in the first trimester group (48%), compared to women in the third (34%) and second trimester (32%). In addition, normocytosis remained more than 50% in all groups of women. Regarding MCH, hypochromia was higher in women in the second trimester (66%) than those in their first and third trimester (62% and 54%, respectively). Comparison of the different proportions showed no significant difference (p> 0.05). As for biochemical parameters, a highly significant difference (p=0.003) was recorded only for higher than normal chlorine levels.
Hematological and biochemical parameters |
Stages of pregnancy (trimester) |
P-values |
|||||
|---|---|---|---|---|---|---|---|
First |
Second |
Third |
|||||
Hematocrit (%) |
n |
% |
n |
% |
n |
% |
0,30 |
MCV (fl) |
24 |
48 |
16 |
32 |
17 |
34 |
0,22 |
MCH (pg) |
31 |
62 |
33 |
66 |
23 |
46 |
0,15 |
Creatinine (mg/l) |
4 |
8 |
7 |
14 |
3 |
6 |
0,35 |
HDL cholesterol (g/l) |
5 |
10 |
6 |
12 |
4 |
8 |
0,70 |
LDL cholesterol (g/l) |
38 |
76 |
38 |
76 |
40 |
80 |
0,30 |
ASAT (UI/l) |
1 |
2 |
0 |
0 |
1 |
2 |
0,44 |
ALAT (UI/l) |
2 |
4 |
1 |
2 |
2 |
4 |
0,76 |
Total protein (g/l) |
29 |
58 |
26 |
52 |
31 |
62 |
0,34 |
Total bilirubin (mg/l) |
22 |
44 |
16 |
32 |
23 |
46 |
0,26 |
Conjugated bilirubin (mg/l) |
43 |
86 |
46 |
92 |
48 |
96 |
0,22 |
Calcium (mg/l) |
5 |
10 |
13 |
26 |
7 |
14 |
0,10 |
Sodium (meq/l) |
35 |
70 |
29 |
58 |
26 |
52 |
0,20 |
Potassium (meq/l) |
8 |
16 |
2 |
4 |
3 |
6 |
0,09 |
Chloride (meq/l) |
39 |
78 |
45 |
90 |
29 |
58 |
0,05 |
Table 4 Proportions of hematologic and biochemical biomarkers in pregnancy
MCV, Mean corpuscular volume; MCH, Mean corpuscular hemoglobin; ASAT, Aspartate aminotransferase; ALAT, Alanine aminotransferase; n, Number of subject observed in each group; a, b, c, Statistically different value for p < 0.05.
Comparison of the proportions of the other biochemical parameters showed no significant difference. Nevertheless, the table IV revealed normal serum creatinine, ASAT, ALT, conjugated bilirubin and calcium levels at all trimesters of pregnancy.In contrast, this table reported that more than 50% of the studied population had LDL hypocholesterolemia and total hypoproteinemia. The proportions below normal were decreasing in sodium and chlorine at all the stages of pregnancy. At the level of HDL cholesterol, a normal proportion of 52% was recorded in the first trimester, against 52 and 56% respectively in the second and third trimester. As for potassium, the increasing proportions of hyperkalemia were 50%, 62% to 60%, respectively for the first, second and third trimester.
The observed proportions of glycemia, total cholesterol, and triglycerides by stage of pregnancy are shown in Figures 2 (A–C). It showed that 100% of women had normal blood glucose in the first trimester, compared to 98% for women in the second and third trimesters of pregnancy Figure 2 (A). As a result, the comparison within each trimester of the proportions observed showed very highly significant differences (p <0.001), normal proportions compared with the high proportions. In terms of total cholesterol, normal proportions were 56% in the first and third trimester, compared with 52% in the second trimester Figure 2 (B). In addition, by comparing within the same trimester the proportions observed, very highly significant statistical differences (p <0.001), normal proportions compared to the abnormal proportions are revealed. As for triglycerides Figure 2 (C), the normal proportions decreased according to the stages of pregnancy (90%, 84% and 76%, respectively for those being in the first, second and third trimester). Moreover, the comparison of the proportions between them, within each trimester, presented very highly significant statistical differences (p <0.001), normal proportions compared to the abnormal proportions. However, increasing but not significant hypertriglyceridemia is observed Figure 2 (C).
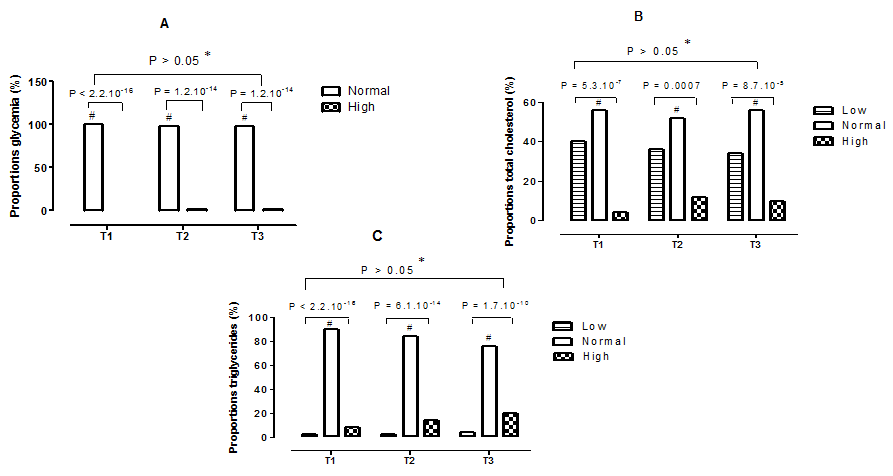
Figure 2 Proportions of some biochemical parameters during pregnancy. T1,First trimester of pregnancy; T2, Second trimester of pregnancy; T3, Third trimester of pregnancy; A, Proportions of glycemia; B, Proportions of total cholesterol; C, Proportions of triglycerides; *, No statistical difference (p > 0.05) between trimesters for the same type of proportion; #: Statistical difference (p <0.05) between the proportions observed in the same trimester.
The monitoring of nutritional parameters in the blood is very important especially for women during pregnancy. It helps to assess their nutritional status and inflammatory status .6,7 Therefore, the study on the variation of blood biomarkers in pregnant women at different stages of pregnancy in the Abobo area (Abidjan) was conducted. This study revealed some alterations in some hematological and biochemical parameters. At the hematological level, the average values of the blood count decreased significantly at all stages of pregnancy, and remained below the reference values. However, mean values of red blood cells remained normal for women in the first and third trimesters of pregnancy. According to,21 this variation found in our study may be due to a slight increase in erythrocyte volume following the stimulation of erythropoietin production by various hormones. In this study, the high proportions of hemodilution and anemia seen in women in the first trimester increased in those in the second and third trimesters of pregnancy. The hemodilution observed in our study could be explained by the increase in plasma blood volume during pregnancy. Indeed, several researchers including,21–23 reported that plasma volume of blood is almost double what it was before pregnancy. According to,21,24 the increase in plasma volume is due to the presence of progesterone and estrogen secreted by the placenta during pregnancy. These cause the release of renin, which stimulates the mechanism of aldosterone-renin-angiotensin, resulting in sodium retention resulting in an increase in plasma volume. Like hemodilution, the study reported a high prevalence of anemia of 36%, 44% and 56% respectively in women being in their first trimester, second and third trimester of pregnancy. This high frequency has also been reported to varying degrees by,19 who found 81.4%, 59.7% and 81.9% respectively in the first, second and third trimesters of pregnancy. In the same vein as our study, the World Health Organization estimated at 38.2% the global prevalence of anemia among pregnant women, and 57% in Côte d'Ivoire.25 In addition, nutritional deficiencies of vitamin B, iron, iron stores and viral infections are the factors that promote anemia, according to several authors.14–17,26 As a result, the high prevalence of anemia observed in our study could be explained by a nutritional deficiency of iron and vitamin B.27 In fact, the micronutrient volume of the foetus would become twice that of the mother.27 It could also be due to malaria, which is an endemic infection in our country.28 Anemia in the mother can lead to variable consequences ranging from maternal morbidity, preterm delivery to infant mortality.26,29,30 However, according to the stages of pregnancy, different types of anemia were distinguished in pregnant women, including normochromic normocytic anemia, normochromic microcytic anemia, hypochromic normocytic anemia, and hypochromic microcytic anemia. The prevalence of the latter type of anemia is higher among pregnant women in their first and second trimesters of pregnancy (20% and 16% respectively in the first and second trimesters of pregnancy). Similar results of anemia have been reported by,14,16,17 whose work focused on HIV-positive and HIV-negative pregnant women. In addition, our study reported the disappearance of normochromic microcytic anemia in the second and third trimester, and a significant increase in normochromic normocytic anemia compared with other types of anemia in the third trimester of pregnancy. In the same vein,31 reported, on the one hand, the absence of normochromic microcytic anemia and, on the other hand, a predominance of normochromic normocytic anemia in pregnant women. In terms of biochemical parameters, the average values of conjugated bilirubin decreased successively in each trimester of pregnancy, while remaining within the normal range. However, this decrease in combined bilirubin was significantly different (p=0.01), compared with the average value in the first trimester. This decrease may be due to the use of medication and the influence that pregnancy may have on the variation of some parameters. Indeed, the high hemodilution during pregnancy could influence the concentration of bilirubin. In 1996,32 reported a decreased conjugated bilirubin in pregnant women during the second and third trimesters of pregnancy. Conversely, the mean values of potassium recorded were increasing. In women in their first trimester of pregnancy, the average potassium was normal. On the other hand, for women in the second and third trimesters, this average was higher than the reference value. Also, comparing these averages with each other, showed a significant difference (p=0.02). Indeed, the potassium in the body is mostly located in the cells (98%). According to,33 hyperkalemia could be explained by several phenomena, including tissue degradation (rhabdomyolysis, hemolysis). Similar results have been reported by.34,35 According to these authors, hyperkalemia can cause disorders of the heart rhythm of variable importance, as well as significant risks of atherosclerotic morbidity. In addition, this study found an increase significant hyperchloraemia in women in the third trimester (30%), compared with those being in the first (14%) and second (4%) trimesters of pregnancy. In the same sense, reported a significant increase in chlorine among women in the first and second trimesters of pregnancy.
The study of blood biomarkers in pregnant women made it possible to highlight all the modifications of the biological parameters assayed. Indeed, anemia was the most observed hematological change. This study also revealed a significant change in biochemical parameters. Pregnancy is an important phase of a woman's life. Therefore, a greater knowledge of vitamin and immunological changes during this phase would contribute to the proper follow-up of this stage of life.
We would like to thank the managers of Abobo South General Hospital for accepting to host this study in their institution in collaboration with the Nangui Abrogoua University. Our thanks also go to the members of the medical analysis laboratory of the South Abobo General Hospital, especially Mr. Koudougou Traoré Moussa, head of services for facilitating access to the hospital and his laboratory and Mr. Koué Bi Honorat, a biologist technician for his assistance during the determination of blood parameters. Our thanks are also extended to all the women who have agreed to participate in this study and Dr Oussou Jean-Baptiste, assistant in Physiology Pharmacology and Phytotherapy Laboratory of Nangui Abrogoua University for his invaluable help in translating this article.
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.

©2018 Aristide, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.