International Journal of
eISSN: 2574-9889


Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) is a lethal disorder of pulmonary development. Patients present with severe respiratory distress and persistent pulmonary hypertension usually refractory to treatment and often associated with other malformations of cardiac, gastrointestinal, or urogenital systems. We report an ACDMPV newborn with persistent pulmonary hypertension with progressive hypoxemia refractory to treatment, duodenal stenosis secondary to annular pancreas, and intestinal malrotation. ACDMPV diagnosis was made based on histopathological evaluation of postmortem lung biopsy and the genetic study that showed a point mutation in the FOXF1 gene.
Alveolar capillary dysplasia (ACD) was first described in 1947, as a lethal disorder of lung development. In 1991, misalignment of pulmonary veins was observed to define Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV).1 To date, over 200 ACMPV cases have been reported in the literature with 10% having a family association.2 ACDMPV is characterized by a failure of the formation and growth with subsequent decrease in the number of pulmonary capillaries that have abnormal contact with the alveolar epithelium, thickening of the muscular wall of the small arterioles, with muscularization of the intraacinar arterioles sometimes accompanied by arterioles that share the same adventitial layer.3,4 These histopathological features can be present in both lungs or in a pulmonary lobe.5 ACDMPV patients present persistent pulmonary hypertension within the first hours of life, generally refractory to treatment with positive pressure ventilation, nitric oxide, sildenafil and even with extracorporeal membrane oxygenation,6 which determines varying degrees of lability and severity with a fatal course in the early postnatal period.7
Between 50 to 80% of ACDMPV patients have associated malformations of gastrointestinal system with intestinal malrotation being the most common (22%), in addition to annular pancreas (11%), imperforate anus (8%), duodenal atresia (5%), duodenal dilation proximal to the pancreas (3%), absent spleen, abnormal placement of the anus and other malformations (3%).8 Also cardiac and genitorurinary malformations, diaphragmatic hernia, and phocomielia have been observed.2,3,9 Cardiac abnormalities include patent ducts arteriosus (46%), hypoplastic left heart syndrome (19%), atrioventricular septal defect (16%), ventricular septal defect (14%), atrial septal defect (11%), aortic coarctation (8%), Tetralogy of Fallot (5%) being the most common8. At a vascular level, poor position of the pulmonary veins adjacent to pulmonary arteries, deficient capillarization of gas exchange membranes, and arterioles with increased muscle, have been described. Urogenital malformations include hydronephrosis (24%), renal pelviectasis (14%), hydroureter (11%), hypospadias (5%), and others.8
These alterations have been associated with mutations in the FOXF1 gene located on chromosome 16q24.1. The diagnosis is currently made through both histopathological study usually obtained at lung autopsy and genetic analysis of the FOXF1 gene.
Newborn female was born at 37 weeks 5 days as a product of a second pregnancy, with prenatal diagnosis of duodenal atresia and esophageal stenosis (Figure 1). Due to a previous cesarean section and rupture of the membranes, an emergency caesarean section was performed. Birth weight was 2100 grams APGAR scores were 8–9 at…min. A thoracic-abdominal radiograph was performed, showing thorax without alterations and an image suggestive of duodenal atresia (Figure 2). An echocardiogram CIA 0.5, PCA: 0.2X0.3 showed mild dysplasia of aortic and pulmonary valves. Abdominal laparotomy at 24hours of life, revealed duodenal stenosis secondary to annular pancreas, intestinal malrotation and non-meconium plastic peritonitis without evidence of perforation. The defect was corrected by adhesiolysis, duodeno-duodenal anastomosis and incidental appendectomy.
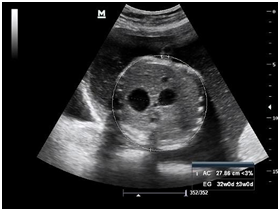
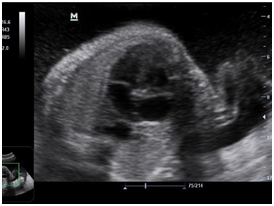
At 48 hours of age, the proband presented an episode of apnea that required positive pressure ventilation with a mask bag without remission of the episode, initiating invasive mechanical ventilation; However, desaturation persisted and the need to increase FiO2, and was changed to high frequency ventilatory mode. Due to a clinical picture compatible with pulmonary hypertension, an echocardiogram was performed, revealing severe pulmonary hypertension with an important shunt from right to left through ductus arteriosus. Therefore, orally sildenafil was initiated (max 2mg/kg/day), but no response was obtained and nitric oxide was added at 20ppm.
After 72hours, the patient presented hemodynamic decompensation with hypotension, and inotropes were started: dobutamine, dopamine and norepinephrine. A control echocardiogram was performed with pulmonary artery pressure (PAP) of 76mmHg. Initially, it resulted in a good response to ON, ventilatory parameters were reduced and it was switched to IPPV mode, and inotropic support was reduced, at 12 hours echocardiogram of control with PAP of 66mmHg.
However, on day … episodes of desaturation appeared with an increase in FiO2 and ON up to 40ppm, and the patient remained under sedation. On the sixth day, milrinone was added to improve perfusion at the pulmonary level and norepinephrine was reinitiated to increase systemic blood pressure. In addition, a dose of surfactant was administered, chest radiography showed bilateral parahiliar interstitial infiltrate, peripheral pulmonary vasculature increased, and decrease in left cavities (Figure 3). Nevertheless, within a few hours she had bradycardia and hypotension, and a new echocardiogram was performed, evidencing right chambers failure and an advanced resuscitation was initiated with drug administration and cardiac massage without obtaining a response, causing patient´s death. The transfontanelar scan was normal. A karyotype was done with a normal report (46,XX).
Parents only authorized a lung biopsy in which at the macroscopic level hypoperfusion and a single left pulmonary lobe were evident (Figure 4). At the microscopic level, important alveolar collapse was reported, isolated foci of intra-alveolar luminal protein deposition, as well as occasional desquamation of pneumocytes, additionally isolated foci suggesting a certain pulmonary immaturity with alveoli of tubular appearance, accompanied by bronchial structures with mural hyaline cartilage and persistence of high epithelia at the distal level, presence at the alveolar level of metaplastic cuboidal pneumocytes.
In addition, the presence of alveolar walls thickened with vascular congestion and hemorrhage mural thickening and diminution of luminal caliber were observed. The presence of muscularized intracinar arteries and decrease of capillaries in relation to alveolar walls was evidenced. The veins were dilated and surrounded by edematous stroma. With this finding, persistent pulmonary hypertension and congenital alveolar capillary dysplasia were concluded. DNA was extracted from the lung tissue and the genomic DNA was used for the PCR amplification of two coding exons of the FOXF1 gene (mRNA reference sequence: NM_001451.2). DNA sequencing analysis was performed using the Sanger method. A pathogenic, heterozygous missense variant: c.290G>A (p.Arg97His) was identified (Figure 5). Analysis of parental samples showed that the mutation arose de novo.
ACD/MPV is a fatal disorder that should be considered in neonates with idiopathic persistent pulmonary hypertension whose symptoms recur after various treatments. The main clinical manifestation is the early onset respiratory distress. The respiratory distress in our patient started at 48hours of age which is compatible with the report of Sen et al.,6 the age of respiratory distress was in the first 24hours. The corrective surgery was performed after 24 hours of life and the patient was stable shortly after, the pulmonary hypertension started and it was irreversible. It is noted that most cases reported were born at term and appropriate weight for gestational age as our patient was. More than one congenital anomaly was usually present in the cases reported.
In a case series in 2017, Nadal RR11 of 6 patients with ACDMPV, the most common associated congenital anomalies were; cardiovascular (patent ductus arterioles, patent foramen ovale, ventricular sept defect, auricular sept defect, partial anomalous pulmonary venous return draining into the right atrium, zygote continuation of inferior vena cava into superior vena cava, drainage of renal and adrenal veins to hepatic vein (HV), HV continuation to right atrium, and pulmonary atresia) and multi systemic as hydrops, pearl effusion, ascites, and anasarca. Other congenital anomalies associated are gastrointestinal, genitourinary, musculoskeletal that were reported by Sen et al.5 Our patient had duodenal stenosis secondary to annular pancreas, intestinal malrotation, and non-meconium plastic peritonitis, also a right to left shunt through a patent ductus arterioles, and on the autopsy an abnormal lobation of the lungs was noted.
Histopathologically, all reported cases of ACDMPV are characterized by decreased number of pulmonary capillaries located away from the alveolar epithelium, alveolar wall thickening, medial hypertrophy of small pulmonary arteries with muscularization of distal arterioles and immature lobular development.8,9 Malposition of the small pulmonary veins is considered pathognomonic, although it is not detected in every case.10 In 2009, Stankiewicz et al.,2 demonstrated inactivating mutations in the FOXF1 gene on chromosome 16q24.1 in patients with ACDMPV. The FOXF1 gene belongs to the family of FOX transcription factors and plays an important role in pulmonary morphogenesis and the development of the intrinsic vasculature as well as the cardiac, gastrointestinal, and urogenital development in the fetal period. It is necessary for the formation of the vasculature to regulate endothelial genes and the signaling of vascular endothelial growth factor.11
To date, approximately 40% of all ACDMPV cases have mutations in the FOXF1 gene, the majority are de novo mutations, but familial inheritance and siblings’ involvement were also reported in some cases.12,14 In 2013 Sen P et al.,14 reported an updated list of all the known FOXF1 mutations, conducted a study of more than 90 children diagnosed with ACDMPV and their relatives, finding that most of the cases are sporadic. There are 41 listed mutations in the FOXF1 gene. In this report we find a case with the same mutation of our patient (c.290G>A), there are no other cases reported with this mutation.
In this report, we described an Ecuadorian newborn with ACD/MPV carrying a de novo mutation in FOXF1. She showed severe pulmonary hypertension refractory to treatment, accompanied by intestinal malrotation and annular pancreas. Diagnosis of ACD/MPV might be challenging for several reasons, definitive diagnosis depends on pathologic features of lung tissue on autopsy or less common from ante mortem lung biopsy; and the lack of clinical knowledge could also contribute not to suspect and under diagnose this entity. For that reason, using and being able to perform the FOXF1 genetic testing could improve the diagnostic accuracy as shown in our case.
This case report is important for us, since is the first clinic-pathologically and genetically confirmed case of ACD/MPV in our country, highlighting the importance of the clinical suspicion and the possibility of FOXF1 testing, although it is not done in our country, for diagnosis in neonates with refractory pulmonary hypertension.
None.
The author declares there are no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.