International Journal of
eISSN: 2470-9980


Research Article Volume 2 Issue 6
1Department of Veterans Affairs Medical Center, Laboratory of Bacterial Pathogenesis, Georgia
2Department of Microbiology and Immunology, Emory University School of Medicine, Georgia
Correspondence: Susu Zughaier, Department of Microbiology and Immunology, Emory University School of Medicine, VAMC, 151-I, 1670 Clairmont Road, Atlanta GA 30033, Tel 404-321-6111, Fax 404-320-2210
Received: September 18, 2016 | Published: October 7, 2016
Citation: Zughaier SM (2016) NGAL Suppresses Immune Responses to Meningococcal Capsular Polysaccharide-Based Vaccine. Int J Vaccines Vaccin 2(6): 00051. DOI: 10.15406/ijvv.2016.02.00051
Meningococcal capsular polysaccharide (CPS) based vaccines are recognized by Toll-Like receptors TLR4 and TLR2 on the surface of immune cells. The recognition of CPS polymers by the innate immune system is a prerequisite for optimal protective and adaptive immunity. Neutrophil gelatinase-associated lipocalin (NGAL) has a physiological role as iron carrier protein and exerts antibacterial function by scavenging bacterial siderophores. In response to cellular perturbation NGAL is highly induced and it exerts anti-oxidant and immune modulatory effects. However, elevated levels of NGAL are associated with various chronic diseases. It is not known whether elevated NGAL levels impact immune responses to vaccines. In this study, I investigated if NGAL exerts immune modulatory effect on macrophage innate immune responses induced by meningococcal CPS vaccine. These CPS polymers were extracted from the endotoxin-deficient meningococci serogroup B (CPS-lpxA) and from serogroups A, B, C and W-135. Here I show that NGAL suppressed TNFα and CXCL10 (IP-10) release from human macrophages induced with meningococcal CPS polymers suggesting that NGAL impaired the recognition of meningococcal vaccine. Further, cellular iron levels have been shown to modulate TLR4 signaling. Therefore, the effect of LCN2, the murine homolog of human NGAL, on murine RAW264 macrophage immune responses was examined in presence of the mammalian siderophore 2,5dihydroxybenzoic acid (DHBA). Here I show that LCN2 significantly decreased mouse TNFα release from RAW264 cells stimulated with meningococcal CPS-lpxA doses in the presence of LCN2 protein. The addition of the mammalian siderophore alone also resulted in significant reduction in TNFα release. However, the pre-incubation of LCN2 with 2,5DHBA led to even more reduction in TNFα release suggesting that NGAL bound siderophore 2,5DHBA modulates cellular signaling and innate immune responses possibly by chelating intracellular iron. Together, the data suggest that elevated NGAL dampens immune responses to meningococcal vaccines which may lead to suboptimal protective immunity.
Keywords:neisseria meningitides, vaccine, infection, capsular polysaccharide, ngal,
macrophage, immune response
BSA, bovine serum albumin; CD4, cluster of differentiation 4; CPS, capsular polysaccharide; CXCL10, chemokine x chemotactic ligand 10; DHBA, dihydroxybenzoic acid; ER Stress, endoplasmic reticulum stress; FBS, fetal bovine serum; HLA, human leukocyte antigen; IP-10, interferon-inducible protein 10; LCN2, lipocalin 2; NGA, neutrophil-gelatinase associated lipocalin; TLR, toll-like receptor; TNFα, tumor necrosis factor
Pattern recognition receptors like Toll-Like Receptors (TLRs) highly expressed in macrophages, sense the presence of invading pathogens via their pathogen associated molecular patterns (PAMPs) and elicit innate immune responses to curb and clear the infection.1 Neisseria meningitidis is a leading cause of bacterial meningitis and fulminant sepsis.2 Meningococcal capsular polysaccharides (CPS) are a major virulence factor and form the basis of vaccine. The recognition of meningococcal CPS polymers by TLR2 and TLR4 induces potent inflammatory mediators release such as cytokines and chemokines from macrophages.3 This innate immune response is a prerequisite to inducing protective adaptive immune responses.2
Upon infection macrophages induce variety of host defense responses to prevent pathogen survival. Neutrophil gelatinase-associated lipocalin (NGAL) is a 25 kDa protein also known as siderocalin, lipocalin 2, and 24p3 that has a physiological role as iron carrier protein. In addition, NGAL exerts antibacterial function by scavenging bacterial siderophores.4 Therefore, NGAL is constitutively expressed at basal physiological levels and is up-regulated upon cellular perturbation and infection.5‒7 Structurally, NGAL is a member of the lipocalins family which transport small hydrophobic molecules such as lipids, steroids, bile and retinoids, share a common tertiary structure, the so called “lipocalin fold” characterized by an eight strand antiparallel β-barrel fold with a hydrophobic calyx that contains the ligand binding site.8,9 Physiologically, NGAL is an iron-trafficking protein that binds the mammalian siderophore component 2,5 dihydroxybenzoic acid (2,5DHBA),10 is expressed and secreted upon stimulation by many cells and also acts as a growth and differentiation factor in various tissues, mainly in the kidney.11 NGAL is equally important for host defense by starving bacteria of iron, thereby preventing bacteria from establishing infection. NGAL-deficient mice are more susceptible to bacterial infections12 and NGAL is required for pulmonary host defense against Klebsiella pneumonaie .13 But, very high levels of NGAL are detrimental to host.7,14‒18
Elevated NGAL level has been observed in various diseases ranging from infections to chronic inflammation, renal dysfunctions, and cancer, and hence is proposed as a biomarker for these diseases.7,19‒24 Serum NGAL is significantly increased in critically ill children with septic shock and acute kidney failure.25 NGAL is released usually in complex with pro-metalloproteinase-9 (MMP9) and protects MMP9 from proteolytic degradation. NGAL is also released from epithelial cells as a holo protein.26 Further, NGAL protein can circulate in a plasma pool alone or can form a complex with MMP9.27 Circulating levels of NGAL/MMP-9 complex have been correlated with bad prognosis and pathologies including inflammatory conditions and cancer.19 Therefore, chronic NGAL elevation above physiological levels is associated with disease state and detrimental. However, the mechanisms by which elevated levels of NGAL contribute to disease remain poorly understood.
Recently I reported that inflammation combined with endoplasmic reticulum (ER) stress synergistically induced dramatic increase in NGAL expression in macrophages.5 High level of NGAL led to dysregulated innate immune responses and exerted immune suppressive effect in macrophages.5 It is not known if elevated NGAL levels can impact immune response to vaccines. NGAL levels are elevated in elderly population as well as in low birth weight infants28,29 and both age groups are known to have lower response to vaccines. The aim of this study is to investigate whether NGAL impacts the immune recognition of meningococcal CPS-based vaccine by macrophages. Here I demonstrate that NGAL suppresses macrophage immune responses induced by meningococcal CPS polymers. Therefore, the data suggest that NGAL exerts an immune suppressive effect which reduces the recognition of meningococcal vaccine.
Reagents
RPMI 1640 medium, Dulbecco’s modified Eagle medium (D-MEM), fetal bovine serum (FBS), penicillin/streptomycin, sodium pyruvate and nonessential amino acids were obtained from Cellgro Mediatech (Herdon, VA). TNFα and CXCL10 (IP-10) ELISA kits were from R&D Systems (Minneapolis, MN). Recombinant human NGAL protein and its murine homologue LCN2 were purchased from R&D Systems (Minneapolis, MN). 2,5-Dihydroxybenzoic acid (2,5DHBA) was purchased from Sigma.
Cell cultures
THP-1 human macrophage-like cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown in RPMI 1640 with L-glutamate supplemented with 10% FBS, 50 IU/ml of penicillin, 50µg/ml of streptomycin, 1% sodium pyruvate and 1% non-essential amino acids. Culture flasks were incubated at 37oC with humidity and 5% CO2. Murine macrophages (RAW 264.7 from ATCC) were grown in D-MEM supplemented and incubated as noted above.
Macrophage treatment with recombinant NGAL
Freshly grown THP-1 cells were adjusted to 106 cells/ml and treated with 100ng/ml of recombinant human NGAL protein that was endotoxin free and produced in mammalian cells or 100ng/ml of sterile BSA (Sigma). From cell suspensions, 250µl aliquots were dispensed into each well at 250 × 103 final cell density in the designated 96-well plates prior to stimulation with 20µg/ml of meningococcal CPS polymers prepared as previously described.3,30 The plates were incubated overnight at 37°C with 5% CO2 and humidity. In some experiments and prior to stimulation with meningococcal CPS-lpxA, LCN2, the murine homologue of NGAL, was added to RAW264.7 macrophages (1 x 106 cells/ml) at 200ng/ml alone or liganded (pre-incubated) with 2,5 dihydroxybenzoic acid (2,5DHBA) at 2µg/ml final concentration. Working CPS-lpxA concentrations (ranging from 20 to 1.25µg/ml) were made in duplicate wells using sterile PBS by serial fold dilutions in 96-well tissue culture plates at 50µl final volumes. Supernatants from stimulated cells were harvested and stored at -20°C for cytokine release measurements.
Cytokine release quantification
The cytokines TNFα and CXCL10 (IP-10) released from THP-1 cells following CPS stimulation, were quantified by DuoSet ELISA (R&D Systems) as previously described.31,32 Similarly murine TNFα was measured in supernatants from RAW264 macrophages using ELISA method.
Statistical analysis
Mean values ± SD (standard deviation) and p values (Student t- test) of three independent determinations were calculated with Microsoft Excel software.
NGAL suppresses macrophage innate immune responses induced by meningococcal CPS-based vaccine
Monocyte-derived macrophages are effectors cells that play a very important role in innate immune defense by uptake and killing of invading pathogens. Macrophages also act as antigen presenting cells and therefore play an important role in connecting innate and adaptive immunity.33 Upon perturbation, peripheral monocytes7 and neutrophils secrete NGAL.34 In order to understand the pathological consequences of elevated circulating NGAL levels on macrophage innate immune function, specifically, the ability to recognize meningococcal vaccine, I used recombinant human NGAL protein. Since serum NGAL levels in healthy donors were found to be ~50ng/ml;7 I used rhNGAL at 100ng/ml to mimic elevated NGAL levels in serum. To this end, human THP-1 macrophage-like cells were treated with recombinant human NGAL protein (rhNGAL 100ng/ml) or sterile BSA prior to stimulation with highly purified meningococcal CPS polymers (20µg/ml) obtained from the endotoxin-deficient serogroup B CPS-lpxA3 and from other meningococcal serogroups A, B, C, W1353,30 and further incubated overnight. Cytokine and chemokine release was measured in the supernatants of stimulated macrophages using the ELISA method. Here I report that rhNGAL but not BSA, suppressed CPS-lpxA induced cytokine production in THP-1 macrophages as demonstrated by decreased production of the inflammatory cytokine TNFα and inhibition of release of the pro-inflammatory chemokine CXCL10 (IP-10) (Figure 1). The effect of NGAL on the recognition of other meningococcal CPS vaccine serogroups was also investigated. THP-1 macrophages treated with high dose of rhNGAL (100ng/ml) prior to stimulation with meningococcal CPS obtained from serogroups A, B, C and W135 resulted in reduced TNFα (Figure 2A) and abolished CXCL10 (IP-10) release (Figure 2B). Recognition of meningococcal CPS by macrophages is important for inducing robust innate immune response that enhances antigen presentation and promote adaptive immune responses.35,36 CXCL10 plays an important role in T lymphocyte recruitment and proliferation.37 Therefore, the data presented here show that NGAL suppresses the immune recognition of meningococcal capsular polysaccharides vaccine and suggest that elevated NGAL levels may dampen or lead to suboptimal adaptive immune responses to meningococcal vaccine.
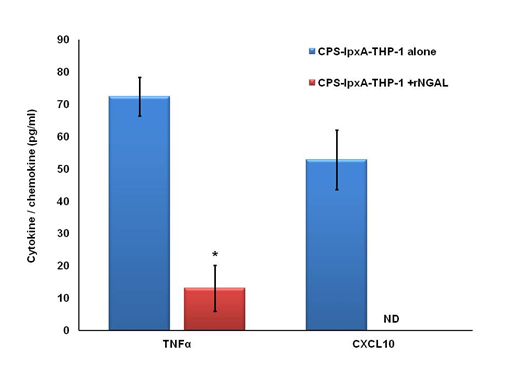
Figure 1 Recombinant NGAL suppressed CPS-lpxA induced cytokine release from macrophages. Cytokine TNFα and chemokine CXCL10 (IP-10) release from human THP-1 cells induced with the endotoxin-deficient meningococcal CPS-lpxA (20µg/ml) in the presence or absence of rhNGAL (100ng/ml). TNFα and IP-10 release was measured in the supernatants of stimulated THP-1 cells using the ELISA method. Bars represent mean ± SD of triplicate readings of each sample.

Figure 2A Cytokine TNFα release from human THP-1 cells induced with meningococcal CPS from serogroups A, B, C and W135 (20µg/ml) in the presence or absence of rhNGAL (100ng/ml).
Figure 2B Chemokine CXCL10 release from THP-1 induced as in panel A. TNFα and CXCL10 release was measured in the supernatants of stimulated THP-1 cells using the ELISA method. Bars represent mean ± SD of triplicate readings of each sample.
Figure 2 Recombinant NGAL suppressed other meningococcal CPS serogroups induced cytokine release from macrophages.
The molecular mechanism underlying NGAL’s suppressive effect is not known. NGAL plays a critical physiological role in intracellular iron homeostasis by trafficking iron-bound mammalian siderophore.10 The basic component of the mammalian siderophore is 2,5DHBA which has been found to protect from oxidant stress by capturing free iron.10 Further, cellular iron levels have been shown to modulate TLR4 inflammatory signaling.38,39 Therefore, the immune suppressive effect of NGAL alone or in the presence of the mammalian siderophore component 2,5DHBA on CPS-induced TNFα release from macrophages was investigated. Specifically, the effect of the murine homolog of human NGAL known as LCN2 on murine RAW264 macrophage innate immune responses was examined in presence of 2,5DHBA. Here I show that rLCN2 significantly decreased mouse TNFα release from RAW264 cells stimulated with meningococcal CPS-lpxA doses (ranging from 20 to 1.25µg/ml) in the presence of rLCN2 (200ng/ml) protein (Figure 3). The addition of the mammalian siderophore alone also resulted in significant reduction in TNFα release. However, the pre-incubation of LCN2 with 2,5DHBA led to even more reduction in TNFα release suggesting that NGAL bound siderophore component 2,5DHBA modulates cellular signaling and innate immune responses possibly by chelating intracellular cellular iron. Together, the data presented here show that NGAL suppresses the immune recognition of meningococcal CPS vaccine which impedes innate and adaptive immune responses.
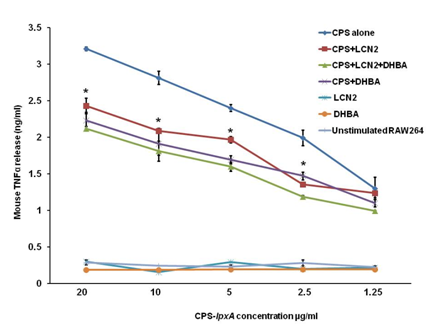
Figure 3 NGAL bound siderophore dampens immune recognition of meningococcal CPS. CPS-lpxA induced TNFα release from murine macrophages RAW264 (1 x 106 cells/ml) was reduced in presence of recombinant LCN2, the murine homologue NGAL., LCN2 (200ng/ml) was pre-incubated alone or with the mammalian siderophore component 2,5DHBA (2µg/ml) for 30min at 37°C, prior to addition to macrophages and overnight stimulation with CPS-lpxA (20µg/ml) derived from the endotoxin-deficient meningococci serogroup B [3]. TNFα release was measured in the supernatants of RAW264 cells using the ELISA method. Bars represent ± SD of triplicate readings of each sample.
*P values are <0.001 calculated using Student t-test.
Effective vaccines elicit protective immune responses with immune memory. The desired protective immune responses of vaccine can be hampered by many host factors such as senescent immune system, dysfunctional immune cells and abundance of inhibitory molecules. In addition to host factors, the nature and the immunogenicity of vaccine antigen impacts the desired protective immune responses. Meningococcal CPS polymers that form the basis of vaccine are recognized by TLR4 and TLR2 in macrophages as reported by Zughaier.3 Robust innate immune recognition of vaccine antigen is a prerequisite for optimal adaptive immune response. NGAL as a lipocalin molecule plays an important role in cellular homeostasis and in inflammation and detoxification processes resulting from innate immune system activation.20 NGAL is the iron carrier lipocalin protein that exerts immune suppressive effects possibly by chelating iron consequently dampening cellular signaling pathways. However, the exact molecular mechanism by which NGAL exerts immunosuppressive effect manifested as reduced cytokine release by macrophages is not well elucidated. The consequences of elevated NGAL levels on immune responses to vaccines are not known; therefore, I investigated whether high level of NGAL impacts the recognition of meningococcal CPS vaccine by macrophages. The addition of rhNGAL to monocyte-like macrophages (THP-1) resulted in significant reduction of meningococcal vaccine-induced pro-inflammatory cytokines TNFα and chemotactic chemokines like CXCL10. The data presented here indicate that elevated NGAL levels suppress macrophage immune responses induced by meningococcal CPS vaccine. In support, recent literature demonstrated that treatment of adipocytes and murine macrophages with exogenous NGAL led to a 30% reduction in LPS-induced cytokine production, which represents an immunosuppressive effect.40 The study showed that NGAL acted as an antagonist to TLR4-MD-2 activation but did not elucidate the mechanism by which NGAL exerted its immune suppressive effect.40 Devireddy and co-workers reported that NGAL is induced and secreted in IL-3-deprived pro-B cells leading to apoptosis in a wide variety of leukocytes, as part of its function as a growth and differentiation factor.41 However, this apoptotic effect was delayed since cellular viability was greater than 85% after 24hrs of exposure to NGAL. NGAL-induced apoptosis was not significant earlier than 60hrs of exposure.41 These findings suggest that NGAL’s immune suppressive effect seen at 24hrs of LPS exposure is not due to cell death which lends strong support to my data that NGAL reduced active cytokine release from viable macrophages after 16hrs of exposure to meningococcal CPS vaccine. Other literature proposed that NGAL modulates inflammatory responses by sequestering neutrophil chemo-attractants like IL-8, N-formylated tripeptides and leukotriene B4.42,43 The data presented in this report show that NGAL treatment abolished CXCL10 (IP-10) release from macrophages stimulated with meningococcal CPS vaccine. CXCL10 is critical for recruiting and enhancing T cell proliferation, thus important for eliciting protective immune responses. Further studies are needed to investigate the direct binding of NGAL to small chemotactic chemokine like CXCL10.
Upon TLR4 activation by LPS two signaling pathways are induced: a TLR4-MyD88-NFkB-dependent signaling pathway that leads to release of TNFα, IL-1β, IL-644 and other pro-inflammatory cytokines, and a TLR4-TRIF-IRF3-dependent signaling pathway that leads to rapid type I interferon (IFNβ) release,31,45 consequently inducing IFN-dependent chemokines like CXCL1031 which plays an important role in connecting innate and adaptive immune responses. Recently, altered iron homeostasis in mice was reported to selectively impair the TLR4-TRIF-IRF3 signaling pathway.39 Another study demonstrated that severe iron deficiency blunted LPS-induced pro-inflammatory cytokines release from macrophages, suggesting that adequate intracellular iron levels are required for optimal TLR4-mediated responses.38 My data show that NGAL treatment in macrophages blunted CXCL10 release which is in agreement with these studies. I also show that NGAL bound to the mammalian siderophore 2,5DHBA significantly dampened TLR4-mediated signaling as evidenced by the significant reduction in TNFα release. Since iron homeostasis has been shown to modulate inflammation, it is possible that NGAL dampens TLR4-mediated responses by chelating intracellular iron. I previously reported that cellular perturbations induced by inflammation and ER stress dramatically and synergistically upregulated NGAL expression and dyregulated cytokines and iron homeostasis in macrophages.5 Taken together, the suppression of vaccine-induced signaling pathways by NGAL suggests that the exerted suppressive effect is modulated by intracellular iron homeostasis. Therefore, elevated levels of NGAL may lead to suboptimal immune responses to vaccines.
Furthermore, recent study reported that TLR5-mediated sensing of gut microbiota is required for optimal antibody response to seasonal Influenza vaccination.46 The study demonstrated that TLR5-deficiency in mice as well as the absence of microbiota in germ –free mice reduced antibody responses to Influenza vaccine. These TLR5-mediated responses are dependent on macrophages and not dendritic cells.46,47 The authors previously showed that TLR5-deficency in mice lead to significant increase in LCN2 i.e. NGAL levels.47 Since NGAL exerts immune suppressive effects, I would argue that the elevated levels of LCN2 in TLR5-deficient mice may resulted in the observed reduced antibody responses to Influenza vaccine. Further, NGAL is reported to induce immune tolerance as it increased the expression of HLA-G on CD4+ T cells in a dose and iron-dependent manner, and increased the expansion of CD25+FoxP3+T regulatory cells.48 These studies lend strong support to my current data presented here that elevated NGAL levels suppress immune responses to meningococcal vaccine.
Lipocalins in domestic animals as well as plant lipocalins are known allergens to human that cause allergy by inducing IgE-mediated Th2 hypersensitivity (reviewed in details.49 These common allergens adopt the typical lipocalin structure with the β-barrel fold and the ligand binding pocket that has high 3D homology to human NGAL. For example, cow milk lipocalin Bos d 5 is reported to bind liganded iron and manipulate T helper CD4 cell response.50 The authors concluded that apo Bos d 5 allergen (devoid of iron) induced Th2 response and inflammation, whereas holo form of Bos d 5 (containing iron) induced immune suppression.50 Not surprising then that lipocalins induce sensitization and allergies, skew and dysregulate immune responses. In summary, NGAL exerts immune suppressive effect on macrophages and reduces the recognition of meningococcal CPS vaccine possibly by chelating cellular iron (Figure 4). Although elevated NGAL level in response to cellular perturbations may act as anti-inflammatory it also dampens immune responses to vaccines leading to suboptimal protective immunity.
Figure 4 Schematic model for how NGAL suppresses the immune recognition of meningococcal CPS-based vaccine. The recognition of meningococcal CPS polymers by TLR4 and TLR2 in macrophages leads to cytokine and chemokine release. NGAL is the iron carrier protein that binds to its receptor and shuttles liganded intracellular iron. The addition of NGAL to macrophages reduced immune responses induced by meningococcal CPS polymers, possibly by chelating intracellular iron, consequently reducing cytokine (TNFα) and chemokine (CXCL10) release.
Therefore, elevated NGAL dampens immune responses to meningococcal vaccine leading to suboptimal protective immunity.
The effect of lower levels of NGAL on macrophage immune responses is not known and has not been tested which a limitation of my study presented here. Future studies should examine whether normal or lower levels of NGAL would impact immune responses to meningococcal CPS-based vaccine.
This work was supported by grants from Emory-Egleston Children’s Research Center and Center for Pediatric Nanomedicine of Emory + Children’s Pediatrics Research Center to S.M.Z. The author is grateful to Dr. Seshu Gudlavalleti for providing meningococcal vaccine-grade capsular polysaccharides. The content of this article is solely the responsibility of the author and does not necessarily represent the official views of the Department of Veterans Affairs.
Susu M. Zughaier is supported by a Development Research Program grant from the Henry M. Jackson Foundation through NIH grant 1U19AI113170-01 (Ann E. Jerse, PI, Uniformed Services University, Bethesda, MD), and she gratefully acknowledges William M. Shafer (Department of Microbiology and Immunology, Emory University School of Medicine), without whose generous support this work would have not been accomplished.
Author declares there are no conflicts of interest.
None.

©2016 Zughaier. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.