International Journal of
eISSN: 2470-9980


Short Communication Volume 2 Issue 2
Dalhousie University, Canada
Correspondence: Srinivas Garlapati, Dalhousie University, 1107-1094 Wellington street, Halifax, B3H 2Z9, NS, CA, Tel +1 902 456 5643
Received: October 16, 2015 | Published: May 16, 2016
Citation: Garlapati S (2016) Decision Analytic Modeling for Cost-Effectiveness Evaluation of Adjuvanted and Non-Adjuvanted Flu Vaccines in the Elderly 65+. Int J Vaccines Vaccin 2(2): 00028. DOI: 10.15406/ijvv.2016.02.00028
The use of immunization strategies to prevent disease and escalation of healthcare costs is well documented. The use of flu vaccines among the senior population has been found to reduce health care costs by preventing influenza complications like pneumonia, increased susceptibility to bacterial infections, use of antibiotics and anti-viral drugs, and need for care in intensive care settings, and death. The currently used non adjuvanted vaccines (TIV) have been found to be having reduced efficacy in the adults due to immune-senescence. The use of adjuvanted influenza vaccines (ATIV) has shown to overcome immune-senescence and further increase the efficacy of flu vaccine strategies in the 65+ population. However, the ATIV is more expensive compared to TIV. The higher costs associated with ATIV can make it difficult for health policy makers to support their use in an already stretched out health care system. Random clinical trials have shown that though ATIV do not reduce the infections but have been shown to reduce complications associated with such infections. Hence, there is a need for methods to help policy makers to show the cost-effectiveness of ATIV by using current available vaccine efficacy, and health care costs data associated with influenza complications. This paper uses decision analytical model tree to simplify cost-effectiveness analysis for health policy decision makers to use of adjuvanted influenza vaccines in the population 65+.
Keywords: influenza; vaccines; seniors; TIV; ATIV; cost-effectiveness; decision analytical modeling
Hemorrhagic septicemia is an economically important bacterial disease of cattle and buffaloes.6 The disease remains a significant obstacle to sustainable livestock production in most parts of tropical Asia and Africa. It is caused by Pasteurella multocidaa natural inhabitant of the mucosal surfaces of upper part of the respiratory tract of ruminants, and under predisposing environmental or management conditions which constitute stress for the animals such as transport (shipping fever), marketing, change of feed, climate or ventilation.1 The disease is per acute, having a short clinical course, involving severe depression, pyrexia, submandibular edema, and dyspnea, followed by recumbency and responsible for 70% above bovine mortality.2
Diagnosis of the Pasteurellosis has been traditionally based on history, clinical signs, necropsy findings and isolation of the causative organism. The organism can be identified by classical microbiological tests particularly biochemical tests.3 Blood smears and impression smears of the organs stained by Leishman’s stain can be useImmunization is one of the most cost-effective public health initiatives that save lives, prevent suffering, provide longevity and better quality of life.1 Immunization not only provides medical benefits both to the individual and the society but also been recognized as best investments in healthcare based on extensive analysis of both cost-savings2 and cost effectiveness.3 Influenza causes severe illness in young children, people with chronic illnesses especially in persons 65+. Hence, individuals 65+ are important targets of seasonal flu immunizations because of immune-senescence, increased prevalence of chronic diseases, higher risk of flu related emergency department visits, hospitalization in intensive care units, and higher risk of deaths. The flu complications in 65+ individuals lead to increased burden on already stretched healthcare resources. Cost benefit studies have pointed out that flu immunization in adults saves $45 on each dollar spent.4
Current state of Influenza vaccines in the adults
Currently used flu vaccines (TIV) have low efficacy (20%) in adults >65 because of immune-senescence. Vaccine efficacy is defined as the proportion of the number of people mounting a protective immune response out of all the persons vaccinated. Hence, there is a need for more efficacious vaccines that are also safer at the same time. The efficacy of the flu vaccines has been improved by use of adjuvants like AS03 (GSK) and MF59 (Novartis). But these adjuvanted vaccines (ATIV) are more expensive with costs ranging from $8.9-18.525 compared to $7.49 of TIV.6 The efficacy of ATIV has been found to be around 40%. Recent large-scale random clinical trials conducted in several countries have shown that ATIV were 40% more efficacious than the current standards of care (TIV). One of the major issues associated with flu vaccines is the need for repeated annual vaccination because of seasonal changes of circulating flu virus strains. Hence, there is a need to study the cost effectiveness of flu vaccines in the persons aged 65+ in Canada. Also, there are only few studies that have assessed the efficacy and effectiveness in elderly populations, generating data of good quality. Hence, this study tries to bring into context the current large scale RCTs and meta-analytical studies and use these data to develop decision analytical modeling using decision trees.
The review of the Cochrane database indicated that the currently available data on the efficacy and effectiveness of influenza in the 65+ individuals is inconclusive.7,8 The available evidence suggests that the best effectiveness of influenza vaccine is for persons in long- term care facilities and the worst for those in community dwelling people. Moreover, any conclusions regarding the effects of flu vaccines for the elderly cannot be drawn because of the poor quality of evidence.9 The current literature was searched for adjuvanted vaccines that are more efficacious than the conventional vaccines.
A Medline search for reports between 2009-2012 with the search terms “elderly”, “65years”, “seasonal”, “influenza”, “vaccine” and “adjuvant” identified 7 studies of efficacy or effectiveness estimates of adjuvanted seasonal flu vaccines. Importantly only 4 of those were randomized clinical studies and only one study was done in community settings.10 This study is the largest RCT comparing non-adjuvanted ad non-adjuvanted vaccines giving the first efficacy estimate in the elderly population. The study was comprehensive done in 43,802 elderly in 15 countries including Canada.10 Moreover, the study was done in real community settings involving ambulatory and healthy persons aged 65+ compared to earlier studies done in institutionalized frail individuals. In this study the efficacy of ATIV was compared to TIV without a placebo group because of ethical reasons. The study was done over a two year period over two flu seasons.10 The participants were followed by telephone; home and site visits and samples for laboratory diagnosis were collected from participants reporting flu like symptoms. The results of this indicated that ATIV demonstrated higher efficacy in reducing infections but the results were not found to be significant. However, ATIV was efficacious in reducing the number of cases of pneumonia, hospital admissions, and deaths. Also, ATIV was effective against Influenza A infections. The major drawback of this study was not considering economic evaluation with vaccine efficacy and effectiveness.10
Economic evaluation of adjuvanted influenza vaccines in the elderly
Medline search using the terms “elderly”, “65years”, “seasonal”, “influenza”, “vaccine”, “adjuvant” and “cost effectiveness” displayed only one result indicating the paucity of cost-effectiveness studies in using adjuvanted flu vaccines in the elderly. A recent cost-effectiveness study demonstrated that the replacement of current influenza vaccine with adjuvanted vaccine in the elderly was highly cost effective11 (Incremental Cost-Effectiveness Ratio of $2111/per QALY gained). The study further projected that adjuvanted influenza vaccines would confer substantial benefits to both vaccinated and unvaccinated individuals and would be economically attractive in comparison to other currently used strategies. The enhanced cost effectiveness of using ATIV in 65+ individuals are very significant with respect to the WHO benchmarks which indicate that health care programs are effective if one can buy life years at a cost of less than per capita gross domestic product, which is $40,000 for Canada.12 These projections have found to hold even under assumptions of minor enhancements in efficacy of adjuvanted vaccines.
A number of studies showed that immunization reduces the costs associated with infections, physician visits, emergency department visits, intensive care admissions, and influenza related cases of pneumonia and deaths.6,11,13
The activity of decision analysis needs is different from the perspective of measurements of RCTs. While the RCTs are focused on different types of measurements, the decision models are concerned with informing specific decisions. The activity of decision analysis is based on the primacy of identifying an appropriate course of action from various alternatives for a specific group of people. This information should be based on currently available evidence. The identification of the preferred course of action is based on the alternatives rather than on individual parameters. Moreover, such decisions are taken under conditions of uncertainity.14 In this study, a decision tree was generated based on influenza vaccine efficacy of new adjuvanted vaccine (ATIV) with the currently used non-adjuvanted vaccine. A recent large scale RCT showed that though ATIV do not reduce the rates of infections with flu but significantly reduce the number of deaths and pneumonia in the adults 65+.15 The complications associated with pneumonia lead to escalation of costs of care associated with emergency department visits, hospitalization in intensive care unit, and treatment with anti-virals. Moreover, in the 65+ individuals the most likely outcome is death. But the cost of ATIV is more than TIV and hence there is a need to compare the cost effectiveness of these alternatives. It should be kept in context that ATIV is twice more efficacious (40%) than TIV (20%). The cost benefits are compared assuming 30% of the persons not protected getting infected. It is further assumed that 3% of the persons infected develop complications, needing to be treated in hospital setting.16 The cost of each complication associated with influenza with respect to the 65+ elderly has been derived the Canadian institute of health information (CIHI) patient cost estimator.17
The purpose of the present cost effective analysis is to investigate if the use of more expensive adjuvanted vaccine (ATIV) is cost-effective compared to currently used vaccine for preventing influenza related complications. The decision tree has been structured (Figure 1) using three options of not vaccinating and vaccinating with TIV or ATIV. Each of these options in turn has three outcomes of getting protection from infection (vaccine efficacy), infection without complications, and infections resulting in complications. It was further assumed that the costs associated with mild infections to be minor with respect to physician office visit and treatment with over the counter drugs.
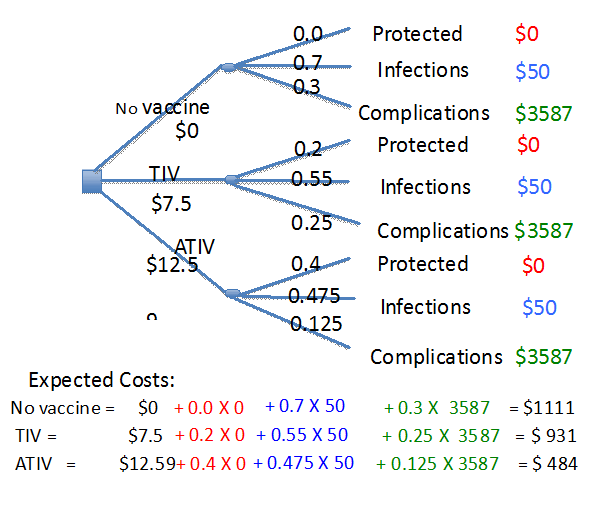
Figure 1 Decision analytical tree comparing immunization with TIV and ATIV with no immunization. The costs have been calculated using 40% efficacy of ATIV compared to 20% efficacy of TIV in the adult 65+ individuals.11 The complications among 65+ individuals immunized with TIV and ATIV is assumed to be 25 and 12.5% in individuals immunized with TIV and ATIV respectively.13 The healthcare costs with respect to complications among 65+ individuals have been derived from Canadian institute of health information (CIHI) patient cost estimator.17
The costs associated with each complication were extracted from the patient cost data retrieved from the CIHI for individuals 65+ in Canadian settings.18
The branch probabilities calculated were shown as indicated in the tree. The costs and branch probabilities were made only for one year because of the seasonal nature of flu infections and need for repeat annual immunization. The uncertainity assumed here is the effect of the transfer of immunity from previous year’s immunization or infection.
The Decision tree was used in this study because they show all decisions to be made in a sequence. The logic of decisions and outcomes are easy to follow. The strengths of decision trees are that they are intuitive and visually effective ways of decision analytical modeling. They are also quick and easy to generate results. Moreover, decision trees are appropriate when they are limited number of health states as in the case of influenza. It was also preferred to use decision trees for relatively short model durations as in the case of influenza. It should be remembered that influenza vaccines need to be administered every year and there is limited protection from previous vaccination or infections. The acute nature of influenza also makes the decision trees amenable to study their cost effectiveness.
The calculation of expected costs are shown in Figure 1 indicate that the highest costs incurred by the system were for not being vaccinated. However, the cost incurred for the ATIV was the lowest. The current care costs were in the intermediary range. As shown Figure 1, immunization with TIV saves only $180 compared to a saving of $527 with ATIV. These changes are because of ATIV being more efficacious compared to TIV. Therefore, the saving in healthcare costs very effectively covers the higher cost of ATIV. Hence, we can state the use of adjuvanted vaccines is cost effective compared to the non-adjuvanted vaccines.
Further analysis of the cost savings per dollar spent on the vaccine regimen (Figure 2) indicates that the cost effectiveness per dollar spent on TIV and ATIV were around 24 and 49.8 compared to not vaccinating at all. The cost effectiveness is more pronounced when we compare ATIV with the current vaccine in use, which amounted to a saving of about $87.6 per dollar. The enhanced efficacy of ATIV will also lead to enhanced herd immunity among the elderly and reduced societal costs associated with influenza.18 Moreover, the projections used in this study were also found to be robust under wide ranging sensitivity analysis.11
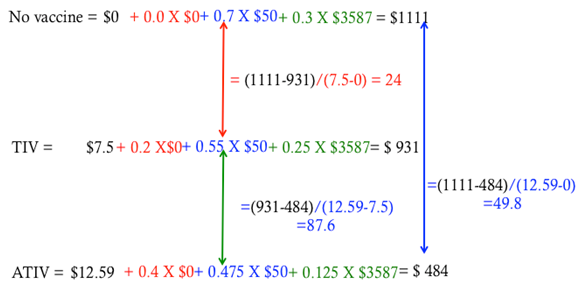
Figure 2 Cost-effective analysis of non-adjuvanted (TIV) and adjuvanted flu vaccines (ATIV) compared with no vaccination scenario. The cost effectiveness has been calculated as savings (difference between no vaccine, TIV, and ATIV as the numerator) per dollar spent on vaccine (difference between the cost for no vaccine, TIV, and ATIV as the denominator).
The adjuvanted influenza vaccines are cost effective in the 65+ elderly populations in Canadian settings. The RCT data used in this analysis was done in the community settings as opposed to earlier trials done in frail institutionalized population. It should be remembered that the efficacy of vaccines is dependent on close match between the predicted virus strains used in the vaccine and actual circulating virus strains. The RCT results also show the adjuvanted do not reduce the rate of infections in the elderly but reduce the costs associated with infections by reducing the number of flu related complications in the vaccinated individuals. This is due to immune-senescence and reduced levels of antibodies in the elderly but the protection against severity is elicited by such immune responses. The decision tree model used in this study indicates that the adjuvanted flu vaccines are more cost effective than non-adjuvanted vaccines. The cost savings using ATIV is shown to offset the increased cost of adjuvanted vaccines in the 65+ individuals. Decision tree analysis offers a simplistic way of using current data on vaccine efficacy, and costs of vaccine, and cost of infections to the healthcare system to look at cost effectiveness of influenza vaccines. Hence, decision tree analysis offers an effective methodology to healthcare managers and policy decision makers at the provincial and federal levels to study cost effectiveness of immunization strategies in the adults. But the availability of current information on healthcare costs of flu infections and complications will be critical in making projections on the effective use of healthcare dollars. This is important with respect to changing demographics of the Canadian population and increased proportion of elderly and efforts associated with escalation of healthcare costs.
The author wishes to thank the staff and faculty of School of Health Administration, Dalhousie University for their inputs and support.
Author declares there are no conflicts of interest.
None.

©2016 Garlapati. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.