International Journal of
eISSN: 2573-2889


Review Article Volume 7 Issue 1
Postgraduate program in biotechnology, Federal University of Alfenas, Brazil
Correspondence: Debora Souza dos Santos, Postgraduate program in biotechnology, Federal University of Alfenas, Brazil, Tel +55 75 991988244
Received: October 07, 2024 | Published: October 16, 2024
Citation: Santos DS, Santos BR, Walter FAJ, et al. Study of non-silent and deletion mutations of SARS-CoV-2 in relation to viral behavior in the infectious process. Int J Mol Biol Open Access. 2024;7(1):122-131. DOI: 10.15406/ijmboa.2024.07.00181
SARS-CoV-2 is a 30,000-nucleotide single-stranded RNA virus encoding structural, non-structural and accessory proteins. Its origin is uncertain, with hypotheses suggesting evolution from bats or pangolins. Genetic recombination events between strains may have resulted in the emergence of SARS-CoV-2, making it capable of infecting humans and increasing its transmissibility. Its mutability rate is similar to that of other coronaviruses, however, due to the occurrence of the pandemic and the propagation clusters, there was an intensification in the number of mutations generated, which evolutionarily gave rise to lineages, sublineages, variants and strains, consecutively. Such mutations, especially non-synonymous ones, phenotypically reflect a variation in virulence, associated with factors of transmissibility, pathogenicity, multiplication, immunological evasion that differ from their ancestors, which are accumulating and becoming fixed in the lineages of this sarbecovirus. In this way, this study aims to understand such non-silent mutations by correlating changes to the viral infectious process.
Keywords: COVID-19, lineages, variants, genetic variations, non-synonymous mutations, deletion mutations
SARS-CoV-2 is a positive-sense single-stranded RNA virus (+ssRNA - 5' cap and 3' poly A) belonging to the order Nidovirales,1 that is, enveloped and with 30,000 nucleotides.2 Its genome it has non-translational regions with 14 open reading frames, the ORFs, which encode 4 structural proteins: nucleocapsid (N), envelope (E), membrane (M) and spike (S); 16 non-structural: ORF1a/b, referring to 2/3 of the genome that translates 2 polyproteins: pp1a (Nsp1- Nsp11) and pp1ab (Nsp1 – Nsp16), in addition to 9 accessory proteins, such as a helicase (Nsp13), endoribonuclease (Nsp15), exonuclease (Nsp14), methyltransferase (Nsp16) in addition to other Nsp's: 3a, 3b, 6, 7a, 7b, 8a, 8b, 9b and 9c.3–5 Taking into account the period of registration of the virus, late 2019, and according to the characteristics of the closest common ancestor (MRCA – Most Recent Common Ancestor), it described possible scenarios that triggered the situation that consequently established the discovery of the coronavirus, SARS -CoV-2, in China, among which: the evolutionary predecessor of this betacoronavirus may have been in latent mode in bats, but active in the mutagenic aspect and accumulating these recombinations, which, for some reason, began viral transmission to humans, probably in Huanan market in China; another alternative is that an ancestor with lower virulence had historically already infected humans and the set of mutations over this period of years triggered the episode in China in 2019 after an increase in virulence; and the last circumstance is that an ancestor of this sarbecovirus was already in circulation in intermediate hosts until it randomly became transmissible to humans.6
Despite its uncertain origin, COVID-19 may have arisen from several genetic recombination events, possibly due to different strains infecting a single host at the same time, and SARS-CoV-2 may be the result of this new combination between the virus and bat and the pangolin.6,7 This evolutionary point, recombination, comes both by crossing the limits of the host species through multiple events with different strains linked to different locations, thus generating purifying selection, as well as other areas of the viral genome through an ancestral route, the which led, for example, to the spike (S) protein being able to infect humans and having a greater affinity with ACE2 (human), increasing its transmissibility in relation to its predecessors.7 Other regions of the viral genome have a lower conservative index in the evolutionary chain and tend to generate viral mutations, widely known as variants. They may have different “behaviors” of infectious, transmissibility and symptomatic processes. Linked to this, there is the fact that the origin of this infectious agent, dated in November 2019 by Xia from a phylogenetic tree sampling 83,688 genomes with complete information from the period collected to track its emergence, has not been completely elucidated as to how viral initiation in humans has been established.3 This lack of information on the molecular origin of this sarbecovirus is directly related to the lack of possibility of prevention and scientific preparation for new occurrences,7,8 mainly with the occurrence of variants of concern (VOC) during the pandemic,9 which corroborates greater exposure to “surprises”. Thus, one way to understand the trend of the virus is to base the genomic changes along with the chronological variation of its “behavior”, that is, the biological properties of the non-synonymous mutations of the strains, to understand the infectious variations occurring in SARS- CoV-2, objective of this study.
Study carried out based on an integrative bibliographic review with a qualitative and descriptive bias. The stages of construction of this research were adapted from Martins,10 in which the direction for the construction of the selection of study articles made is through the guiding problem question of the work – in this case, variation in the infectious activity of SARS-CoV-2 – and, after this first moment, articles are selected from the databases and with pre-defined keywords, classification of the collected material, followed by screening to compose the final sample, subject to critical analysis for compilation and writing. Among the pre-defined criteria of the study are: the databases to search for articles: PubMed and Direct Science in the search period from 2020 to 2024.
At the beginning of the pandemic, in 2020, the new virus discovered, SARS-CoV-2, characteristically has a positive RNA single-strand structure, in which the 5' terminus encodes proteins for viral replication and has open reading frames, while the terminal 3' is the carrying site for structural proteins.11 The first region of the viral genome, after the 5' UTR, is ORF1/ab, which corresponds to approximately 66% of the entire sequence and is responsible for encoding 16 non-structural proteins , to the NSPs, while the other parts encode structural proteins: spike (S), membrane (M), envelope (E) that surround the nucleocapsid (N), in addition to accessory proteins: orf3a, orf6, orf7a, orf7b, orf8 and orf10, Figure 1a.11,12 During the infectious process, the SARS-CoV-2 viral genome performs specific functions at different stages of the infection, being crucial for its replication and dissemination. The different parts of the viral genome, together with the proteins they encode, are fundamental to the efficiency of the viral replication cycle. The S protein encompasses three regions: the transmembrane and intracellular region, in addition to the ectodomain, which has two subunits, the S1, responsible for interaction with the human receptor, which involves the NTD (N-terminal) and RBD (Receptor Binding Domain), the latter determining viral transmissibility and cellular tropism; and S2 that performs membrane fusion that comprises a cleavage site other than the one present between the S1 and S2 regions, fusion peptide, cytoplasmic domain, and Heptad 2 (HR2) and transmembrane S repeat regions, Figure 1b.13–16
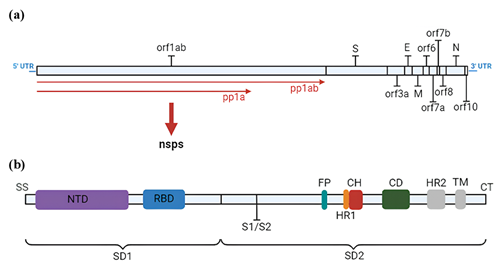
Figure 1 (a) SARS-CoV-2 genome and encoded proteins with identification of the region of the genomic sequence where each is located; (b) SARS-CoV-2 spike protein genome. Caption: orf1ab (Open Reading Frame 1ab); S (Spicle); orf3a (Open Reading Frame 3a); E (Envelope); M (Membrane); orf6 (Open Reading Frame 6); orf7a (Open Reading Frame 7a); orf7b (Open Reading Frame 7b); orf8 (Open Reading Frame 8); N (Nucleocapsid); orf10 (Open Reading Frame 10); pp1ab (Polyprotein 1ab); pp1a (Polyprotein 1a); nsps (NonStructural Proteins); SS (Simple Sequence); NTD (N-Terminal Domain); RBD (Receptor Binding Domain); S1/S2 (S1/S2 Protease Cleavage Site); FP (Fusion Peptide); HR1 (Heptad Region 1); CH (Central Helix); CD (Connector Domain); HR2 (Heptad Region 2); TM (Transmembrane Domain); CT (Cytoplasmic Tail); SD1 (Subdomain 1); SD2 (Subdomain 2).
The RBD domain, among the different species of the Coronaviridae family, has different cellular receptors, SARS-CoV and SARS-CoV-2 have ACE2 (Angiotensin-converting enzyme 2), a component of the RAAS (Renin-angiotensin-aldosterone system) among the betacoronovirus as a receptor, while alphacoronavirus has HCoV- NL63, differing only in terms of affinity level, in which the latter has 10 to 20 times greater than SARS-CoV,13,17 in addition to the possibility of the DPP4 enzyme of the CD26 complex (Adenosine deaminase 2) act as a co-receptor when the virus enters the hosts' airways, similar to MERS-Co-V.18,19 After viral attachment to the host, the S protein is cleaved in the region between S1 and S2 by TMPRSS2 (Transmembrane Serine Protease 2), which may also be caused by lysosomal cathepsins or furins, exposing the S2 fusion peptide.13,20,21 Then, with the release of genetic material and transcription into genomic RNA (important in viral replication) and subgenomic RNA [translating template for membrane, envelope and spike structural proteins in the rough endoplasmic reticulum, directed to the ERGIC (Endoplasmic Reticulum Intermediate Compartment- Golgi) and for excretion in sequence, the association of the envelope protein with the RNA in the ERGIC occurs, generating the nucleocapsid, which favors budding containing structural proteins and gives rise to mature virions that are released by exocytosis.13,17
Since the discovery of the SARS-CoV-2 virus, over the following three years, a significant volume of genomic variations detected in this virus has been observed, which has a mutagenic tendency, at a higher level in the S (spike) protein, which is 4-5 times more changes than the rest of the genome.2,4 For genes in general, in a situation of neutral genetic drift, the rate of substitutions per base is 0.003, that is, 30 nucleotides per genome,2,22 already the daily rate of mutations/genome are approximately 0.05526.23 Based on the structural study of SARS-CoV-2 by Naqvi and collaborators, a minimal change in the genomic sequence of the virus can generate a significant change in the structures of viral target proteins, negatively impacting the effectiveness of previously available medicines.24 In this way It is evident that the pandemic generated impacts that were not geographically uniform, but with defined stages, which present this variation due to several factors that include exposure, infection, health index and death of the population, in addition to taking into account the context of the occurrence of underreporting in this unique period and the significant impacts that variants introduce in this scenario.25
For example, in Brazil, these stages of Covid-19 were defined considering the number of doses of vaccines taken, at least two, mortality, level of incidence and positive diagnostic tests with data taken from Fiocruz's MonitoraCovid-19, a study that listed five moments of the disease in the country, with the first wave in which there was a geographic expansion of the disease across the entire territory, from large centers to the interior, from March to August 2020; the second in the period between September 2020 and January 2021 was stabilization of the transmission level; the third represented a collapse of the system with the highest number of deaths per day, reaching up to 4,000 between February and June 2021; July to November of the same year was marked by the fourth wave, characterized by the impact of the effects of the vaccination wave that began in July, generating a reduction in transmission; and the last, fifth wave, highlighted by the significant increase in the infectious spread of the virus again by the omicron variant, this during December 2021 to March 2022.25 One of the major factors considered to recognize the stages of Covid-19 in the country were the variants, the only parameter without the possibility of control, only reduction through restrictive measures, such as lockdown to prevent spread, since the lower the circulation of the virus, the lower is the probability of more variants emerging,26 in addition to the fact that its primary reproduction (R0) of person-to-person contagion is 2.6, that is, it grows exponentially if isolation measures are not met,27 reinforcing the unpredictability of the effects of the virus to the population.
The strategy adopted by the WHO since the beginning of Covid-19 can be described by the need for countries to prepare by increasing internal emergency response mechanisms so that there is adequate detection, protection of the population and treatment with trained professionals and a community informed about the risks and necessary actions to be taken, in order to reduce transmissibility, supported by innovation and learning, informed by Tedros Adhanom Ghebreyesus in an interview in the initial phase of the pandemic.28 Internally, in Brazil, the strategy adopted by Fiocruz converges with what the WHO established, strengthening the production, dissemination of knowledge and technology as its mission.29 Therefore, during the pandemic, the UN (United Nations), since the decree by the WHO (World Health Organization) of a state of global pandemic on March 11, 2020 and even after the decree of the end of COVID-19 as a global health emergency disease on May 5, 2023, made periodic reports on the disease around the world available on its website, providing information to monitor the progress of the pandemic.28,30 That said, from June 1, 2021, with the emergence of variants, a nomenclature was created to prioritize these mutations according to three biases, monitoring, interest and concern, respectively called VUM (Variant Under Monitoring). in which it requires attention in case it becomes a threat to global public health; VOI (Variant of Interest) are those in which their mutations generate some change in the potential impact on the population or behavior, such as transmissibility and level of infectivity; and VOC (Variant of Concern), a term used for those that fall into the VOI category and also have characteristics that can have a significant impact on the health system by reducing the effectiveness of medications and vaccines, in addition to the possibility of increasing the severity of the disease.31,32 There is also a nomenclature for variants that no longer present risks to public health compared to the others, but does not rule out the possibility of becoming a VOI or VOC again, this group is called previously circulating VOC/VOI.33
In March 2023, so that the sublineages can be considered independently and the possibility of new variants originating from previously circulating VOCs, the working definitions of the variants were updated to more accurately track them, modifying the parameters to that a variant can be considered a VOI or VOC in the nomenclature, in which only the VOCs will remain with the Greek letter naming, the VOIs will be from Nextstrain and Pango. For VOI, it is necessary to present mutations that are known to present changes in transmissibility, immunological evasion, virulence, detectability and susceptibility to medications, in addition to dissemination advantages in relation to other circulating ones, manifesting a prevalence and increasing line of cases over time; For VOI to become a VOC, the disease must worsen, have a significant impact on the health system and reduce the effectiveness of vaccines for severe forms of Covid-19.34 This standardization is essential for understanding, at a global level, the emergence of new variants, lineages and, mainly, strains, a target of global health concern, as shown in Figure 2, in which it is possible to observe this order of study priority by authorities, as they present phenotypic changes in transmission, multiplication and/or pathogenicity that differ from their ancestor.26,33 The viability of this study was only possible due to the support of the countries that carried out the phylogenetic and phylodynamic study, inserted in different platforms, in particular, of public access to Gisaid, with approximately 592 thousand genomic sequences submitted until November 2023, for sharing and access to information generated through evolutionary analysis considering the rate of mutation and substitution in phylogenetic trees.28,35–37
Figure 2 Order of priority for global health concerns, intensified in the region closest to the virus.
According to preliminary analysis, it was possible to observe that the substitution (evolutionary) rates are 1 x 10-3 substitutions per site per year and mutation rates have a high value of approximately 1.2 x 10-3 per nucleotide per year, rates similar to the averages of viruses with an RNA genome and other coronaviruses.28,38–40 Such substitutions, synonymous and non-synonymous, which, respectively, correspond to silent mutations that do not phenotypically alter the proteins and non-silent ones that cause variations, in particular, the non-silent ones are the focus because they specify milder functions or rigorous biological action, but the silent ones are responsible for the evolutionary purifying selection of the virus.41 The strains of highest priority for global health organizations are precisely those that fit or have already fit into the nomenclature made by the WHO for variants of monitoring, interest and concern.42 From this, data relating to these strains was analyzed. in the PAHO report and in scientific articles, as can be seen in Table 1, with information regarding these variants that fluctuate between nomenclatures or can be removed according to the expansion of the disease and new waves that increase viral circulation and modify the scenario.42 In addition, silent and non-silent mutations can be seen, which are responsible for the differentiation and characterization of strains.58
|
WHO label |
Lineage |
Genetics features |
Earliest documented |
Date of designation |
GISAID clade |
Nexstrain clade |
References |
|
Alpha |
B.1.1.7 |
N501Y; P618H; ∆69-70del; ∆144del; A570D; D614G; E484K; P681H; T716I; S982A; D1118H |
United Kingdom, Sep-2020 |
·VOC: 18-Dec-2020 · Previous VOC: 09-Mar-2022 |
GRY |
20I (V1) |
Volz et al.,43 Liu et al.,44 Meng et al.,45 Wang et al.,46 Janil et al.,47 Fu et al.,48 Carabelli et al.,49Aleem, SV et al.50 |
|
Beta |
B.1.351 |
N501Y; E484K; K417N/T; D614G; Δ242-244; R246I; L18F; D215G; A701V; D80A |
South Africa, May-2020 |
·VOC: 18-Dec-2020 ·Previous VOC: 09-Mar-2022 |
GH/501Y.V2 |
20H (V2) |
Wang et al.,46 Fu et al.,48 Aleem, SV et al.,50 Akkiz,51 Korber et al.,52 Tatsi FM et al.53 |
|
Gama |
P.1 |
N501Y; E484K; K417T; D614G; T20N; L18F; T1027I; H655Y; V1176F; D138Y; R190S; P26S |
Brazil, Nov-2020 |
·VOC: 11-Jan-2021 ·Previous VOC: 09-Mar-2022 |
GR/501Y.V3 |
20J (V3) |
Fu et al.,48 Aleem SV et al.,50 Korber et al.,52 Tatsi FM et al.,53 Planas et al.54 |
|
Delta |
B.1.617.2 |
N501Y; L452R; P681R; T478K; D614G; T19R; R158G; D950N; G142D; E156del; F157del; R158G |
India, Oct-2020 |
·VOI: 4-Apr-2021 ·VOC: 11-May-2021 ·Previous VOC: 7-Jun-2022 |
G/478K.V1 |
21A, 21I, 21J |
Fu et al.,48 Carabelli et al.,49 Aleem, SV et al.,50 Korber et al.,52 Tatsi FM et al.,53 Planas et al.54 |
|
Epsilon |
B.1.427 / B.1.429 |
B.1.427: L452R; D614G |
United States of America, Mar-2020 |
·VOI: 5-Mar-2021 ·Previous VOI: 6-Jul-2021 |
GH/452R.V1 |
21C |
Carabelli et al.,49Aleem, SV et al.,50 Planas et al.,54 Lazarevic et al.55 |
|
Eta |
B.1.525 |
E484K; Δ69-70del; A67V; Δ144del; D614G; Q677H; F888L |
Multiple countries, Dec-2020 |
·VOI: 17-Mar-2021 ·Previous VOI: 20-Sep-2021 |
G/484K.V3 |
21D |
|
|
Iota |
B.1.526 |
T95I; D614G; L5F; T95I; E484K; A701V |
United States of America, Nov-2020 |
·VOI: 24-Mar-2021 ·Previous VOI: 20-Sep-2021 |
GH/253G.V1 |
21F |
|
|
Um |
B.1.621 |
T95I; Y144S; R346K; E484K; N501Y; D614G; P681H; D950N |
Colômbia, Jan-2021 |
·VOI: 30-Aug-2021 ·Previous VOI: 9-Mar-2022 |
GH |
21H |
Tatsi FM et al.53 |
|
Zeta |
P.2 |
E484K; L18F; T20N; P26S; F157L; D614G; S929I; V1176F |
Colombia, Jan-2021 |
·VOI: 30-Aug-2021 ·Previous VOI: 9-Mar-2022 |
GH |
21H |
|
|
Theta |
P.3 |
E484K; N501Y; 141-143del; P681H; L241del; L242del; A243del; D614G; V1176F |
Brazil, Apr-2020 |
·VOI: 17-Mar-2021 ·Previous VOI: 6-Jul-2021 |
GR/484K.V2 |
20B/2.484K |
|
|
Kappa |
B.1.617.1 |
L452R; E484Q; P681R; D614G; T95I; G142D; E154K; Q1071H; L452R; P681R |
Philippines, Jan-2021 |
·VOI: 24-Mar-2021 ·Previous VOI: 6-Jul-2021 |
GR/1092K.V1 |
21E |
|
|
Lambda |
C.37 |
L452Q; F490S; D614G; G75V; T76I; R246del; S247del; Y248del; L249del; T250del; P251del; G252del; D253N; T859N |
India, Oct-2020 |
·VOI: 4-Apr-2021 ·Previous VOI: 20-Sep-2021 |
G/452R.V3 |
21B |
Carabelli et al.,49 Aleem, SV et al.,50 Tatsi FM et al.,53 Liang et al.56 |
|
Ômicron parent lineage |
B.1.1.529 |
∆69-70del; N501Y; P681H; T91; P13L; E31del; R32del; S33del; R203K; G204R; D3G; Q19E; A63T; N211del/L212I; Y145del; Y144del; Y143del; G142D; T95I; V70del; H69del; A67V; Y505H; Q498R; G496S; Q493R; E484A; T478K; S477N; G446S; N440K; K417N; S375F; S373P; S371L; G339D; D796Y; L981F; N969K; Q954H |
Multiple countries, Nov-2021 |
·VUM: 24-Nov-2021 ·VOC: 26-Nov-2021 ·Previous VOC: 14-Mar-2023 |
GR/484A |
21K |
Table 1 Main strains of Covid-19, as well as fluctuation characteristics of the WHO nomenclature designation and non-silent mutations present in each of these variants, which confer functional activity different from their ancestor.
In order to understand the changes generated by non-synonymous mutations, Table 2 included all these mutations, location of the genome and their biological properties listed in the literature. These non-synonymous variations are accumulating and fixing themselves in SARS-CoV-2 lineages, increasing the probability of phenotypic changes.58 For example, D614G significantly impacted the dynamics of the disease in the pandemic, appearing in the initial period of the disease's spread and becoming dominant across the world. Apparently, this is a relevant mutation in all VOC variants as it increases the packaging of the spike in the virion and is phenotypically associated with increased infectivity, especially in the upper respiratory tract.60,106 It is notable that, most of these scientifically cataloged genomic variations are located in the spike protein, due to the fact that it is a pharmacological target and plays a crucial role in the first stage of the virus's action, entry into the host cell, which suggests an emphasis on the study of this region of the viral genome, scientifically.58,107
|
Amino acid |
Location |
Biological properties |
Type of mutation |
References |
|
N501Y |
Spike |
Increases ACE2 binding, cellular infectivity in animal models, as well as one of the main determinants of increased transmission and immune evasion |
Not synonymous |
|
|
L452R |
Spike |
Immune evasion and increased Spike affinity with ACE2 that leads to greater infectivity |
Not synonymous |
|
|
S13I |
Spike |
Immune evasion |
Not synonymous |
|
|
E484K |
Spike |
Immune evasion and increased Spike affinity with ACE2 |
Not synonymous |
|
|
T95I |
Spike |
- |
Not synonymous |
|
|
L452Q |
Spike |
Immune evasion, increased affinity with ACE2. In addition to immune resistance, combinatorial action of mutations in L452Q and F490S |
Not synonymous |
|
|
H69-V70del |
Spike |
Increases infectivity, immune evasion. Generates conformational mutation in Spike |
Deletion without frame shifting |
Meng et al.,45 Ahmed et al.,66 Chakraborty et al.,68 Hernández C et al.,70 Ghosh71 |
|
R32del |
Nucleocapsid |
Associated with lower risk of ICU admission and death |
Deletion without frame shifting |
|
|
S33del |
Nucleocapsid |
Associated with lower risk of ICU admission and death |
Deletion without frame shifting |
|
|
P618H |
Spike |
Increased infectivity, transmissibility and immune evasion |
Not synonymous |
|
|
D614G |
Spike |
May influence SARS-CoV-2 infectivity, transmissibility and immune evasion |
Not synonymous |
|
|
W152C |
Spike |
Immune evasion and increased transmissibility |
Not synonymous |
|
|
L18F |
Spike |
Immune evasion |
Not synonymous |
|
|
E484Q |
Spike |
Intensification of cell-cell fusion. Combinatorial action of mutations in E156G/Δ157-158, L452R and E484Q |
Not synonymous |
|
|
F490S |
Spike |
Immune evasion, combinatorial action of mutations in L452Q and F490S |
Not synonymous |
|
|
R203K |
Nucleocapsid |
Increased infectivity |
Not synonymous |
|
|
A570D |
Spike |
Increased infectivity |
Not synonymous |
|
|
Δ242–Δ244 |
Spike |
Immune evasion |
Deletion |
|
|
R246I |
Spike |
Immune evasion |
Not synonymous |
|
|
T20N |
Spike |
Immune evasion |
Not synonymous |
|
|
L241del |
Spike |
- |
Deletion |
|
|
T76I |
Spike |
Increased infectivity |
Not synonymous |
|
|
P13L |
Nucleocapsid |
Reduced interactivity with G3BP1-N |
Not synonymous |
|
|
Q19E |
Membrane |
- |
Not synonymous |
Ahmad et al.,66 Ismail et al.86 |
|
A701V |
Spike |
Association with RBD-N501Y mutation causes increased infectivity |
Not synonymous |
|
|
V1176F |
Spike |
Increased affinity with ACE2 |
Not synonymous |
|
|
D950N |
Spike |
Promotion of membrane fusion, immune evasion |
Not synonymous |
|
|
P681H |
Spike |
Increase in cleavage activity, transmissibility, in addition to the association with the RBD-N501Y mutation causes increased infectivity |
Not synonymous |
Carabelli et al.,49 Chakraborty et al.,68 Zaheer et al.,75 Montaño et al.87 |
|
Q1071H |
Spike |
Function unclear |
Not synonymous |
|
|
Y144del |
Spike |
Immune evasion, increased transmissibility |
Deletion without frame shifting |
|
|
K417T |
Spike |
Immune evasion |
Not synonymous |
|
|
P681R |
Spike |
Transmissibility, promotion of membrane fusion and spike cleavage |
Not synonymous |
|
|
A67V |
Spike |
Change in antigenic structure |
Not synonymous |
|
|
141-143del |
Spike |
- |
Deletion without frame shifting |
|
|
G204R |
Nucleocapsid |
Increased infectivity |
Not synonymous |
|
|
T19R |
Spike |
Immune evasion |
Not synonymous |
|
|
Q677H |
Spike |
Transmissibility, increased infectivity and immune evasion |
Not synonymous |
|
|
L242del |
Spike |
Immune evasion |
Deletion |
|
|
A243del |
Spike |
Immune evasion |
Deletion |
|
|
G142D |
Spike |
Change in antigenic structure |
Not synonymous |
|
|
E31del |
Nucleocapsid |
Associated with lower risk of ICU admission and death |
Deletion |
|
|
A63T |
Membrane |
May affect membrane protein dimer stabilization |
Not synonymous |
|
|
D1118H |
Spike |
Non-significant influence on immune evasion, in addition to increased infectivity and transmissibility |
Not synonymous |
|
|
S982A |
Spike |
Non-significant influence on immune evasion, increased affinity with ACE2 |
Not synonymous |
Ahmed et al.,66 Ghosh,71 Hirawat et al.,76 Gerashchenko et al.99 |
|
P26S |
Spike |
Immune evasion |
Not synonymous |
|
|
F157del |
Spike |
Associated with the E156G, R158del and L452R mutations, it generates greater infectivity |
Not synonymous |
|
|
T716I |
Spike |
Increased infectivity |
Not synonymous |
|
|
D80A |
Spike |
Immune evasion |
Not synonymous |
|
|
D138Y |
Spike |
Non-synonymous mutation that has no clear effects on Spike, despite its importance in defining the lineage |
Not synonymous |
|
|
Y143del |
Nucleocapsid |
Deletion |
||
|
D253N |
Spike |
Associated with D614G and 246-252del leads to increased infectivity and immune evasion |
Not synonymous |
|
|
T859N |
Spike |
Can increase interaction between protomers and induction of conformational variation |
Not synonymous |
|
|
Q498R |
Spike |
Increased affinity with ACE2 and reduced transmissibility |
Not synonymous |
|
|
Q493R |
Spike |
Increased affinity with ACE2 and immune evasion |
Not synonymous |
|
|
G446S |
Spike |
Immune evasion, increased affinity with ACE2 and reduced transmissibility |
Not synonymous |
|
|
K417N |
Spike |
Immune evasion and reduced transmissibility |
Not synonymous |
|
|
G339D |
Spike |
Moderate increase in infectivity |
Not synonymous |
|
|
Q954H |
Spike |
Reduction of infectivity |
Not synonymous |
|
|
L981F |
Spike |
Moderate increase in infectivity |
Not synonymous |
|
|
S373P |
Spike |
Increased affinity with ACE2 |
Not synonymous |
Table 2 Mutations observed in the strains, the region of the viral genome where they are located and the biological properties related to them.
The infectious process occurs according to the viral cycle, composed of the stages of adsorption, penetration, denudation, viral synthesis, maturation and release.108 These virulence processes are linked to several factors, such as immunological evasion, infectivity and transmissibility, which are directly affected by the evolution of the virus, that is, the acquired mutations and the emergence of strains and lineages caused by this.109 In Table 2, it is possible to observe how such biological properties are categorized based on these virulence factors and the fact that Immune evasion is the predominant phenotypic expression in this data set, followed by infectivity and transmissibility. These SARS-Cov-2 strains have acquired significant changes, resulting in different characteristics compared to previous forms, influencing several aspects of epidemiology and immunological response and, mainly, virulence. These genetic variations can affect the transmissibility of the virus, immune evasion and drug resistance, the severity of the disease, the effectiveness of control measures and the host's immune response,110 however, to date there are no studies that prove a significant change in how the infectious process occurs, but it should be noted that other parts of the viral genome are preferable as pharmacological targets, other than the spike (S) protein, due to its high mutagenic index, visible in Table 2.111–113
Despite the occurrence of several non-silent mutations, which generated changes in virulence, with greater exposure in the spike protein region, there are not enough changes to confirm a variation in the infectious process of SARS-CoV-2. This study is something that must be continued, in order, mainly, to be able to trace a mutagenic trend line of the virus so that science can somehow be prepared for possible complications from new worrying variants.
WFA acknowledges the financial support from the CNPq (Brazil) (Process Numbers: 308883/2014-4, 309029/2018-0, and 306298/2022-8).
The authors declared that there are no conflicts of interest.

©2024 Santos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.