International Journal of
eISSN: 2573-2889


Research Article Volume 6 Issue 1
1Advanced Research Center in Medicine (CEPAM - UNILAGO), Union of Great Lakes Colleges, São Paulo, Brazil
2Department of Biology, Institute of Bioscience, Humanities and Exact Science, São Paulo State University (Unesp), Brazil
3University College London (UCL), Division of Surgery and Interventional Science, Royal Free Hospital Campus, UK
4Federal University of São Paulo, Post Graduate Program in Structural and Functional Biology, Unifesp-EPM, Brazil
Correspondence: Sonia Maria Oliani, São Paulo State University, R. Cristóvão Colombo, 2265 - Jardim Nazareth (IBILCE/UNESP), São José do Rio Preto, SP, Brazil, Tel +55 1732212381
Received: December 08, 2023 | Published: December 20, 2023
Citation: da Costa NB, Dias EP,Vieira VR, et al. Protective effects of endogenous and exogenous Annexin A1 on ischemia- reperfusion-derived injuries. Int J Mol Biol Open Access. 2023;6(1):76-81. DOI: 10.15406/ijmboa.2023.06.00156
Ischemia and reperfusion (I/R) injury permeates a variety of diseases, causing significant inflammatory and thrombotic responses. Given its importance, efforts have been made to identify the complex and integrated network of mediators that ensure a timely and spatially regulated inflammatory response, in order to mitigate its deleterious effects on the body. The Annexin A1 protein (ANXA1) and its mimetic peptide Ac2-26 can alleviate the inflammatory process by decreasing adhesion and neutrophil trafficking, inhibiting pro-inflammatory and pro-thrombotic mediators, and regulating the interactions of neutrophil-platelets. In this review, we discuss the anti-inflammatory potential of ANXA1 and its intracellular effects, contrasting the possible long-term permanence of the damage caused by I/R injuries. As a pharmacological approach, the aim is to understand how ANXA1 operates in processes resulting from I/R injuries and its performance as a biomarker and therapeutic target after injury in different tissues and organs.
Keywords: annexin a1, inflammation, ischemia/reperfusion, formyl peptide receptor (fpr), therapeutic target
The study of the role of Annexin A1 (ANXA1) and its mimetic peptide Ac2-26 in the inflammatory process has made considerable progress in understanding their mechanisms of action and effects. ANXA1 (37 kDa protein) was the first member to be characterised within the superfamily of proteins that bind to phospholipids in a calcium-dependent manner.1 This protein was initially named lipocortin due to its anti-inflammatory actions similar to those of glucocorticoids, inhibiting the synthesis of eicosanoids and phospholipase A2 (PLA2), thus affecting the components of the inflammatory reaction and the release of arachidonic acid.2,3
The cPLA2 (cytosolic PLA2) enzyme is predominant in signalling the release of eicosanoids through the arachidonic acid cascade. Therefore, by inhibiting the cPLA2 enzyme, it is possible to prevent the expression of integrins on the surface of neutrophils and interleukins.4 ANXA1 also inhibits the production of cyclooxygenase-2 (COX-2), thus controlling the release of pro-inflammatory factors.5 Several studies using ANXA1 mimetic peptides, particularly the N-terminal Ac2-26 peptides, have demonstrated their anti-inflammatory activity and ability to reduce the severity of damage caused by various injuries, such as epilepsy.6 This reinforces the potential anti-inflammatory effects of these peptides and demonstrates their potential function in the regulation of inflammatory diseases. The ANXA1 protein may also be involved in the resolution and repair of skin lesions. Its expression appears to be dependent on healing time and may act as a biological marker of wound closure.7
In addition, ANXA1 may favour the regeneration of the transplanted tissue by limiting the exacerbation of the inflammatory response at its onset, reducing the expression of cytokines Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Tumour Necrosis Factor alpha (TNF-α), Interleukin-1β (IL-17) and Interferon-gamma (IFN-γ), increasing regenerative factors such as Fibroblast growth factors (FGF) and Transforming Growth Factor-β (TGF-β), as well as modulating angiogenesis through the release of Vascular Endothelial Growth Factor-A (VEGF-A).8–10 Maintaining homeostasis is of fundamental importance for resolving inflammatory processes. In this context, ANXA1 and its peptides inhibit the accumulation of neutrophils at the site of inflammation, reducing the influx of leukocytes and activating neutrophil apoptosis. Furthermore, this study indicates that ANXA1 induces the release of immunosuppressive and pro-resolving molecules.11
The recruitment of cells during the inflammatory process is of paramount importance, but the exacerbation of this process and related responses can cause tissue damage. After an ischemic event, it is necessary to quickly restore tissue reoxygenation. However, this ischemia/reperfusion (I/R) process can exacerbate injuries and the destruction of affected tissues, due to a series of activated inflammatory mediators and an increase in adhesion molecules and platelets.12 In this context, ANXA1 and its mimetic peptides are able to attenuate I/R by reducing leukocyte adhesion and migration,13 inhibiting the release of pro-inflammatory and pro-thrombotic mediators14 and regulating neutrophil-platelet interactions.15 (Figure 1) According to La,17 ANXA1 can also reduce cardiac lesions, acting as a protective factor after this type of injury.16 In recent decades, progress has been made in understanding the mechanisms involved in the initiation and maintenance of experimental I/R. However, only some of these advances have been translated into clinical trials aimed at treating injuries in I/R patients and accelerating recovery of tissue function. It is therefore essential to evaluate the inflammatory events that result from I/R injury in order to study the action of ANXA1 in this process.
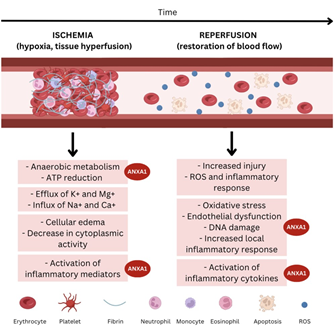
Figure 1 Scheme showing where AnxA1 acts in the process of ischemia-reperfusion (elaborated by the authors).
Inflammatory mechanisms underlying ischemia-reperfusion injuries
The I/R is characterised by the restriction of blood supply to a given organ, followed by reperfusion/reoxygenation of the same, but this process leads to clinical manifestations such as myocardial hibernation, acute heart failure, brain and gastrointestinal dysfunction, systemic inflammatory response syndrome and multiple organ dysfunction.17,18
Pathological mechanisms promote ischaemia, leading to hypoxia and tissue hypoperfusion. The ischaemic state, in turn, induces anaerobic metabolism, which reduces ATP production and causes ion-exchange channels to fail, favouring the dysfunction of potassium and calcium pumps.19 Due to ion dysfunction, cell oedema occurs and enzymatic activity in the cytoplasm is impaired.17 Consequently, the inflammatory cascade and the activation of various mediators occur, with the release of leukotrienes, prostaglandins, prostacyclins and thromboxanes.
Leukotrienes (C4 and D4) cause vasoconstriction, impairing tissue/organ perfusion.
Endothelial prostacyclins have platelet anti-aggregation and vasodilator action via cyclooxygenase. Thromboxane, as a potent vasoconstrictor, aggregator and activator of platelets and neutrophils, causes significant microcirculation impairment.19 When the production of reactive oxygen species (ROS) is prolonged or increased, deleterious events are observed, such as a reduction in the concentration of antioxidants in ischaemic cells, causing oxidative stress with endothelial dysfunction, DNA damage and local inflammatory responses.20 Inflammatory cascades and oxidative stress, associated with intense neutrophil release, induce a cytokine storm leading to muscle oedema, tissue necrosis and cell death.17,20
Mechanisms evoked by endogenous or FPR-binding ANXA1 during ischemia-reperfusion
A family of G protein-coupled receptors (GPCRs) - the formylated peptide receptors (FPR) - can be identified on the surface of some types of human cell types, such as neutrophils, monocytes, macrophages, T cells, endothelial and epithelial cells. The GPCR family has three receptors that have been identified in humans: the formylated peptide receptor 1 (FPR1), FPR2 (also known as ALXR or ALX in humans) and FPR3.21–23 By participating in the recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), FPRs play an important role in the innate immune response.24 Like other members of this family, FPR2 is highly expressed on the surface of endothelial, epithelial and smooth muscle cells, chondrocytes and fibroblasts.25 This receptor can bind to substances such as serum amyloid protein A, lipoxin A4 and, finally, annexin A1, which compete for the receptor binding site.21,26
Probably due to ligand-specific conformational changes in the receptor associated with downstream signalling, FPR2 is capable of triggering effects depending on the ligand. In response to serum amyloid protein A, for instance, FPR2 can trigger a cascade of pro-inflammatory effects.27 It is interesting to note that signal transduction following ANXA1 biding, the ALXR not only triggers the anti-inflammatory cascade,21 but also a transient Phosphorylation of Extracellular Stimulus-regulated Kinase 1 (ERK1; also known as MAPK3 - Mitogen-Activated Protein Kinase 3) and ERK2 (also known as MAPK1). These substances, in turn, are associated with a rapid increase in intracellular calcium.23,28
In pathological inflammatory conditions, the process of restoring homeostasis fails, culminating in a less pronounced inflammation, usually chronic or transient, but recurrent. For resolution to occur, with the restoration of normal tissue or organ function, a series of processes responsible for physiological and protective inflammatory responses must occur in a timely manner and with controlled intensity.29 Identifying and understanding the processes and mediators essential for the progression of inflammation resolution is of great importance. In this context, FPR2 would be a highly relevant target in these harmful conditions since the receptor is involved in many (if not all) of the processes that lead to the resolution of inflammation. For instance, blocking neutrophil extravasation, inducing neutrophil apoptosis, followed by enhanced phagocytosis and efferocytosis by macrophages, promoting non-phlogistic recruitment of monocytes (which differentiate locally into macrophages, contributing to improved clearance efficiency), changes in macrophage phenotype towards resolving profiles and, as a more recent discovery, the induction of stromal cells, which will favour inflammation repair.30–32 In this context, it could be proposed that FPR2 agonists, such as ANXA1, are significantly beneficial by reprogramming relevant target cells in a variety of injured tissues. Therefore, new avenues will be opened up for a better clinical approach among diseases involving persistent inflammation.25
AnxA1 on ischemia-reperfusion in several systems
The anti-inflammatory protein ANXA1 has various effects on different tissues and organs, such as the nervous system, heart, gut, lung and kidney (Figure 2).

Figure 2 Diagram shows the action of AnxA1 in different systems.1
Nervous system
Ischemia/reperfusion (I/R) after ischemic stroke causes excessive brain inflammation through the release of inflammatory cytokines and cell migration to the damaged endothelium, further potentiating tissue injury. The contribution of an enhanced inflammatory response to the pathophysiology of cardiovascular diseases (including stroke) is evident.13 However, to prevent further damage, the inflammatory response must be efficiently modulated. Leukocyte recruitment in the reperfusion process is intense, and ANXA1 in its endogenous circuit can produce significant brain protection. This mechanism can be observed in the inhibition of ANXA1 nuclear translocation by S100A11, a protein secreted by the non-classical pathway, which protects against cerebral ischemia-induced neuronal cell apoptosis.33,34 When associated with S100A11, the nuclear translocation of ANXA1 was reduced, which appears to be involved in increasing cell survival after OGD/R (oxygen-glucose deprivation and reoxygenation). Thus, preventing the ANXA1 translocation in the nucleus may be a protective mechanism that reduces neuron death after ischemic injury.33,35
In addition, ANXA1 and its receptor FPR are responsible for re-establishing homeostasis in injured tissue at different stages of the inflammatory process, either by blocking leukocytes, inhibiting cytokines, promoting apoptosis, stimulating phagocytosis, or diminishing vascular permeability. Targeting FPR2 can modulate these vascular inflammatory responses in a dose- and time-dependent manner.13 A study showed I/R-induced injury to the middle cerebral artery in mice, followed by the administration of ANXA1 and Ac2-26. The effect of this treatment demonstrated a lower incidence of I/R injury compared to the control group. Furthermore, the local inflammatory response was increased in the absence of ANXA1.34
Microglial cells are known to be the main immune cells in the CNS and appear to actively participate in inflammatory processes. The activation of these cells is dependent on several factors, such as prostaglandins, COX-2, nitric oxide (NO), nitric oxide synthase (iNOS) and pro-inflammatory cytokines. During the ischemic injury that occurs in stroke, it is believed that the excessive production of pro-inflammatory mediators contributes to the deleterious process, with consequent neuronal death.36 The interleukins IL-1β, IL-6 and TNFα are essential pro-inflammatory cytokines that, when induced, can excessively activate microglial cells, forming a vicious cycle of pro-inflammatory responses that continually damage neurons and other important structures in the nervous system. On the other hand, IL-4, IL-10 and TGF-β are important anti-inflammatory cytokines that participate in ischemic processes. Pro-inflammatory cytokines are up-regulated, while anti-inflammatory cytokines are down-regulated during ANXA1 translocation in the nucleus of microglial cells. Thus, preventing the nuclear translocation of ANXA1 may regulate excessive and deleterious pro-inflammatory activity.36
Recent studies have demonstrated an interesting mechanism of action for ANXA1 in I/R after stroke. ANXA1 can be modified by a so called “small ubiquitin protein (SUMO)” in a process denominated “SUMOylation” (post- translational modification of endogenous ANXA1), which in turn can promote the microglial polarisation and anti- inflammatory phenotype change after OGD/R, resulting in neuroprotection after I/R.36 Furthermore, it was discovered that the sentrin/SUMO-specific protease 6 (SENP6) acts as a negative regulator of ANXA1 SUMOylation, inducing nuclear translocation and triggering neuronal apoptosis.37 Finally, the group also demonstrated that cerebral ischemia increased the level of SENP6 in microglia in vitro and ex vivo, aggravating the level of brain damage, and its inhibition is capable of driving microglial polarisation and shifting a pro-inflammatory to an anti-inflammatory phenotype. This has shown that the inhibition of SENP6 and, consequently, the deSUMOylation of ANXA1 can provide therapeutic benefits after the I/R process and is a possible mechanism to be used in the treatment of stroke.38
Under normal conditions, ANXA1 makes up the blood-brain barrier (BBB) and promotes its integrity. During the two extremes of life - the prenatal period and old age there are changes in the expression of ANXA1. Therefore, this protein is able to help maintain the integrity of the BBB, while controlling the leukocytes uptake into the brain tissue.39 Recently, an analysis of ANXA1 levels after mechanical thrombectomy in patients who suffered acute ischemic stroke (CVA) was carried out, showing that the reduction in plasma ANXA1 analysed during the onset of CVA in these patients was recovered in about 2-3 days, thus classifying this protein as a post-procedure prognostic marker.40
Heart
Myocardial I/R is recognized as one of the main causes of morbidity and mortality in various heart diseases.41 It is characterised by a series of events, including the marked accumulation of polymorphonuclear neutrophils (PMN) on the endothelium of cardiac blood vessels. These adherent neutrophils release toxic metabolites that cause irreversible damage to the heart.16 Another injury caused to the myocardium after I/R is the increase in ROS and calcium overload.42 The role of ANXA1 in myocardial infarction associated with the inflammatory process caused by I/R has been evaluated. In experiments carried out on rats using recombinant human ANXA1, the protein showed a protective effect on the myocardium against damage caused by I/R, resulting in an anti migratory action of leukocytes. It is believed that this protein acts through a cellular displacement phenomenon, reducing the adhesion of PNMs in the infarcted cardiac tissue.43 Thus, ANXA1 inhibits the infiltration of PNMs, preventing myocardial inflammation, increasing its viability and attenuating infarction. In addition to inhibiting PNM infiltration, ANXA1 has been shown to prevent myeloperoxidase (MPO) activity in myocardial tissues and to accelerate neutrophil apoptosis by activating the STAT3 signalling pathway.41
Gavins,35et al showed that Ac2-26 inhibited acute injury by up to 50% by reducing tissue levels of pro-inflammatory chemokines and cytokines.44 In this case, the protein interacted with the lipoxin A4 or ALX receptor, known as FPR2. Furthermore, Ritche et al (2009) demonstrated that Ac2-26 reduces myocardial damage through the direct effect of the protein on the cardiomyocyte, acting as a metabolic inhibitor by preventing anaerobic glycolysis caused by ischaemia, or through the indirect effect of inhibiting neutrophil activation, thus conferring cardioprotective activity.45 The direct effect of ANXA1 also ensures limited protection for cardiac systolic and diastolic capacity after injury, as well as preventing cardiomyocyte death.46
Gut
Intestinal I/R causes irreversible damage to enterocytes through oxygen depletion which, in turn, releases content in the extracellular matrix and promotes failure of the epithelial barrier, triggering the inflammatory response.47 The loss of barrier function allows the microbiota to migrate to the bloodstream, facilitating systemic inflammation through the release of different inflammatory cytokines. By inducing the I/R process in the mesenteric microcirculation in mice, Ac2-26 abolished the migration and adhesion of leukocytes and extravasation of plasma proteins, whereas in FPR-deficient mice, the anti migratory effect of ANXA1 was reduced, although still effective.48
The effect of AnxA1 or its peptides has not been widely explored in intestinal I/R injury. However, they have shown a protective role in other gastrointestinal inflammatory models, including colitis and gastric ulcers in mice treated with the mimetic peptide Ac2-26, mediated by activation of the FPRL-1 receptor.49 Another murine model of colitis showed that ANXA1 had a pro-resolving effect in a NOX1-dependent manner through the activation of adhesion proteins, epithelial cell motility and wound closure, while ANXA1-deficient mice demonstrated a defective repair, delaying wound healing.50 In addition, local intestinal administration of Ac2-26 peptide in the formulation of polymeric nanoparticles accelerated wound healing in the colon of mice, shedding light on a possible mechanism of this protein as therapeutic target in chronic mucosal lesions, such as Inflammatory Bowel Disease – IBD.51,52
Lung
Among the various organs affected by intestinal I/R injury, the lung is one of the most vulnerable. Guido,54 et al initiated studies showing that Ac2-26 has the effect of reducing lung injury caused by intestinal I/R. It has been argued that this protective effect on the lung parenchyma appears to be due to reduced leukocyte migration to the lung and increased circulation, suggesting a regulatory effect of the Ac2-26 peptide.53 I/R injury is considered one of the main complications following cardiopulmonary bypass (CPB), a method that has been widely used to provide circulatory and respiratory support, along with temperature control during heart surgery. In this context, it has been shown that the application of Ac2-26, which binds to FPR, decreased lung injury after CPB and reduced the levels of pro- inflammatory mediators such as TNF-α, IL-1β, ICAM-1, NF-, κb and p65 in mice.54 Gong,55 et al also observed that mice treated with Ac2-26 after I/R showed an improvement in alveolar capillary permeability.54 This peptide was also responsible for dampening several inflammatory factors, including TNF-α, IL-1β, IL-6, IL-10 and neutrophil elastase, thus restricting the inflammatory process.
Cunha,56 et al demonstrated that acute lung injury (ALI), caused by the administration of endotoxin LPS in mice resulted in leukocyte infiltration, exacerbated production of inflammatory mediators and tissue damage. Treatment with Ac2-26 was able to decrease leukocyte migration, especially in the pulmonary connective tissue and alveoli, contributing to the regulation of inflammation.55 The main cause of lung transplant failure is ALI resulting from I/R. In this context, it has been shown that ANXA1 effectively interrupts the activation of the NF-κB signalling pathway, which is an important transcription factor responsible for the activation of cytokines and chemokines, with a protective effect on the progression of ALI.56
It is known that one of the pathological mechanisms of post-transplant I/R is the excessive production of ROS. It has been shown that ANXA1, by regulating iNOS (inducible nitric oxide synthase) and eNOS (endothelial nitric oxide synthase) levels, is able to inhibit oxygen stress, TNF-α expression, inflammation and apoptosis and, consequently, improve alveolar-capillary permeability.54 Recently, a possible pathway has been proposed in the context of ANXA1 mechanism of action. As a promising therapeutic target to attenuate post-I/R lung injury, it was suggested by Liao,58 et al that the induction of 2-methoxyestradiol, which appears to increase ANXA1 expression, results in a decrease in oedema, neutrophil infiltration, inflammatory cytokines production, oxidative stress, lung cell apoptosis and nuclear translocation of NF-κB in lung injury after I/R 57
Kidney
The most common cause of nosocomial acute kidney injury (AKI) is I/R, which is triggered by a transient restriction of blood perfusion in the tissue parenchyma.58 The kidney is among the most susceptible organs to this type of injury, the mechanism of which, as previously discussed, involves inflammation, leukocyte infiltration, activation of apoptotic pathways, calcium overload, microvascular dysfunction, oxidative stress, and mitochondrial dysfunction.18 Often associated with chronic kidney disease (CKD) and poor prognosis,59,60 AKI has several risk factors in its aetiology and progression, such as heart surgery, hemodynamic instability,61 diabetes, hypertension and use of nephrotoxic drugs.62–65 Although the treatment of I/R injuries has made important progress recently, the control of their exacerbated inflammatory processes continues to represent a significant obstacle. Therefore, the development of new therapies to treat kidney I/R injury is essential.66
In this context, ANXA1 has been shown to play an important role in tissue protection following I/R injury,62,64,67 with less side effects when compared with other classical treatments. Cyclosporine A (CsA) and tacrolimus (FK), drugs widely used as immunosuppressants in the treatment of autoimmune diseases and in post-transplant therapy,68 have significant side effects of nephrotoxicity.69 Their toxic effects are attributed to a drug-induced imbalance in the function of the vessels in the renal circulation, resulting in vasoconstriction of the afferent arteriole, reduced blood flow and ultimately tissue damage.68,70–73 As a result of tissue and cell damage and subsequent I/R, activated macrophages eventually produce vasoactive substances that exacerbate the CsA-induced damage to renal function.74
Studies have shown the relevance of ANXA1 in cases of I/R injuries. The administration of Ac2-26 does not only protect the renal function and tissue structure, but also reduces the expression of endogenous ANXA1.67,75 Experimental methods using renal vascular clamping in rodents corroborated with other studies showing the protective
role of ANXA1 in renal injury. In this type of model, the application of a more recent derivative of ANXA1, the mimetic tripeptide, was able to reduce tubular injuries, decrease serum creatinine levels, limit cell death in renal tubular cells, increase the amount of antioxidant enzymes and mitochondrial DNA repair proteins.76
The protection conferred by ANXA1 has been attributed to its anti-migratory effect on monocytes and macrophages, with a consequent reduction in the influx of these cell types locally in the tissue.64,74 On the other hand, it is believed that the endogenous expression of this protein occurs due to an expected physiological response. The response aimed to suppress inflammation usually fails in the presence of infiltrated lymphocytes that remain in the injured tissue.67 The mechanism behind this increase in endogenous protein is probably associated with transmigrated neutrophils,77 which explains the ability of Ac2-26 to attenuate endogenous ANXA1 expression in tissues, since the peptide is capable of inhibiting leukocyte migration.67
The aim of this review is to explore the role of ANXA1 as a potential pro-resolving mediator in the context of I/R injury in various organs. This treatment aims to facilitate resolution of inflammation by stimulating tissue repair. The peptide Ac2-26, with its chemical stability and lower immunogenicity than the ANXA1 protein, may have several biological advantages in resolving inflammation by reprogramming relevant target cells in ischaemic injury, inhibiting granulocyte trafficking, and stimulating macrophage efferocytosis in vivo. There is growing recognition that the body's differential responses to ANXA1 and Ac2-26, through binding to FPR2/ALX receptors, have anti-inflammatory and thrombotic significance, contributing to organ protection and reducing the damage caused by I/R.
This work was supported by UNILAGO Scientific Development Support Program (PADC-UNILAGO) and Advanced Research Center in Medicine (CEPAMUNILAGO), Union of the Colleges of Great Lakes (UNILAGO), São José do Rio Preto, SP, and the State of Sao Paulo Research Foundation – FAPESP (2019/19949-7), Brazil.
The authors declare that there are no conflicts of interest.

©2023 da, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.