International Journal of
eISSN: 2573-2889


Research Article Volume 4 Issue 6
1Faculty of Veterinary Medicine, Addis Ababa University, Ethiopia
2Department of Animal Science, College of Agriculture and Natural Resource Sciences, Debre Birhan University, Ethiopia
3Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Ethiopia
Correspondence: Jirata shiferaw, Faculty of Veterinary Medicine, Addis Ababa University, Bishoftu, Ethiopia, Tel 0949102441, Fax 251-11-4339933
Received: December 20, 2019 | Published: December 31, 2019
Citation: shiferaw J, Demissie T, Demissie K, et al. Pathological characterization of lesions and bacteriological isolation of causative agents of swine tuberculosis at Bishoftu and Addis Ababa Abattoirs, Bishoftu, Ethiopia. Int J Mol Biol Open Access. 2019;4(6):210-219. DOI: 10.15406/ijmboa.2019.04.00123
Background: TB in swine is natural and virtually common in developed countries where swine is farmed. Although this re-emerging disease is very common in developing country as well; there is scanty information on the prevalence of swine tuberculosis in Ethiopia. Therefore, this study was developed to assess the prevalence of swine tuberculosis by using bacteriological and histhopathological techniques.
Methods/Principal Findings: A cross sectional study was conducted at Bishoftu and Addis Ababa Abattoirs from September 2017 to May 2018 to estimate abattoir based prevalence of tuberculosis in swine, to isolate Mycobacterium species involved and to characterize the lesions. Five hundred and fifty six (556) swine were examined. Tubercle like granulomatous lesion were detected in 19.6% (109/556) of different organs of which 12% (69/556) was in lymph nodes, 5.7% (32/556) in the lungs, 1% (6/556) in the liver and 0.36% (2/556) in the spleen. Based on microscopic alteration 4.7% (26/556) of lesions were tuberculous granulomatous type with central necrosis, calcified foci, epitheloid cells admixed with lymphocyte and connective tissue boundries, 3.6% (20/556) pyogranulomatous and 2.5% (14/556) non necrotic granulomatous lesion without epitheloid cells and connective tissue capsules. The tuberculous lesions found in lymph node were statistically significant (P < 0.05) than in other organs. The multivariable logistic regression analysis showed that old aged swine were more likely to have characteristic tuberculous lesion (OR = 3.14, 95% CI, 1.62-6.09) than younger ones. From the tissue cultured, 7.5% (3/40) yield growth on primary culture media. The observed colony morphology was smooth whitish or yellowish color, sticky, off-white and breaks apart easily and two (5% (2/40)) of these growth were acid fast positive by Zeihl-Neelsen staining technique.
Conclusion/Significance:This study highlights that TB is prevalent in swine and pork can be source of tuberculosis to human when consumed under cooked. Therefore, routine abattoir inspections should be conducted, further molecular and biochemical research to isolate the species of microbacteria is highly recommended to elucidate the type of lesion and its magnitude in different body organs by considering large number of swine.
Keywords: mycobacterium tuberculosis, bacilli, Bishoftu, TB
Abbreviations: SLN, submandibular lymph node; PLN, parotid lymph node; MLN, mesentric lymph node; RLN, retropharyngeal lymph node; LCR, left cranial; LCA, left caudal; RCR, right cranial; RML, right middle; RCA, right caudal; RAC, right accessory lobes of lungs; LR, liver; SP, spleen, TN, tuberculosis
Tuberculosis (TB) is considered as a re-emerging, infectious granulomatous disease in animals and people caused by acid-fast bacilli of the genus Mycobacterium. Tuberculosis occurs frequently in man, domestic and wild animals. The tubercle bacilli are Mycobacterium tuberculosis, the agent of the disease in primates, M. bovis in other mammals and M. avium in birds. Swine are susceptible to all the three types of tubercle bacilli. Swine and dogs are susceptible to both M. bovis and M. tuberculosis. Cats are quite resistant to M. tuberculosis.1 Tuberculosis continues to be an important disease both in humans and animals. It causes morbidity, mortality and economic loss worldwide. The occurrence of Mycobacterium bovis disease in humans, domesticated and wild animals confirms the relevance of this zoonosis. 2–18
Swine are natural hosts for mycobacterial infections including those due to M. bovis.19 The most common cause of swine tuberculosis is Mycobacterium avium, but infection with mammalian tubercle bacilli, including M. tuberculosis, M. bovis, and M. africanum occur coincident with infections of cattle, wildlife, and human beings. The finding of severe, diffuse tuberculous lesions strongly suggests that Nebrodi black pigs are susceptible for Mycobacterium spp. and that they might act as a distributor for these microorganisms. 3 Lesions occurring in swine naturally infected with M. bovis and M. tuberculosis are indistinguishable. Granulomatous lesions are most often found in the cervical, submandibular, and mesenteric lymph nodes, but in advanced disease lesions may also be found in the liver and spleen. Typically, enlarged nodes contain small, white or yellow, caseous foci, usually without any evidence of calcification. Swine with disease due to M. tuberculosis may have similar regionalized lesions with human beings. Swine are particularly susceptible to M. bovis, which is usually acquired from shared grazing or ingestion of contaminated dairy products. This can cause a rapidly progressive, disseminated disease with caseation and liquefaction of lesions. In industrialized countries most tuberculous lesions in swine are caused by bacteria of the M. avium complex (M avium ss hominisuis, M. avium ss avium) and M. intracellulare. Lesions are most often observed in lymph nodes associated with gastrointestinal tract. However, it is important to emphasize some swine may develop progressive disease involving the liver, spleen and other organs of the abdominal and thoracic cavities.4,20
The lesions in swine infected with M. tuberculosis are often associated with lymph nodes of the gastrointestinal tracts. Caseous lesions are most commonly found in the mesenteric or submaxillary lymph nodes; however, microscopic lesions have been observed in the portal and thoracic nodes and in the parenchyma of the lungs.2 The De initiative diagnosis to check whether a certain herd is infected or not requires a clear evidence of the agent based on bacteriological culturing, histopathology and molecular methods.21,22 The main bootle neck for transmission of TB in many country is from wild species and vice versa. According to Pederson et al.,5 infection in white-tailed deer (Odocoileus virginianus) has been followed by infection in cattle in some Midwestern states. Infection has also been documented in feral swine (Sus scrofa) on the Hawaiian island of Molokai and in various European countries, but no large-scale survey of antibody exposure to the bacteria has been conducted in feral swine in the US.6 A major effort to eradicate tuberculosis in swine and in people markedly reduced the incidence of the types of tuberculosis usually seen in swine and people. The serovars of avian type TB found in confinement-raised swine have been found in people may translate into increased concern for TB in both swine and poultry. Shared research in human and animal health can efficaciously speed the development of new diagnostic tests for humans and livestock, and improve TB surveillance, control, and eradication programs.1,20
The main objective of the current study was therefore is the first of its kind in providing baseline information with regard to pathological and histopathological study. The ultimate aim was pathological and bacteriological methods; were used for the diagnosis of swine tuberculosis. Correlation of gross, microscopic and bacteriological findings to increase the sensitivity of TB detection provided the true epidemiological status of the disease and useful information in the swine population in Ethiopia that can be utilized for future research and disease control purpose at national and global level TB control efforts and reducing the risk of its zoonotic significance.
The study was conducted from September 2017 to May 2018 in Addis Ababa and Bishoftu abattoirs. These two abattoirs were selected purposively because they were the only abattoirs slaughtered swine. This animal species has been brought to the abattoirs from different towns of the country.7 The geographical location of Bishoftu is at a latitude and longitude of 8°45′ N and 38°59 ′ E, respectively, with an elevation of 1920 meters above sea level. The reliable seasonal rainfall and temperature prediction would have several advantages for record of disease prevalence and increased agricultural productivity in the country.8 Apart from Addis Ababa and Bishoftu, swine were brought for slaughter to these abattoirs from surrounding Oromia Zone, Sabata and Burayyu which are at most within 60km radii from Addis Ababa. Swine brought from each area were kept under intensive management system, feed poultry manure and left over (disposed carcass of other animals) from abattoirs.
Swine brought to Addis Ababa and Bishoftu abattoirs during the study periods for slaughter were study animals without discrimination of their age, sex, breed and body condition scores. Accordingly, 335 swine, from Addis Ababa and its surrounding and Bishoftu town, brought to Addis Ababa and 221 to Bishoftu abattoirs in total 556 swine were included in the current study. The average number of swine presented to slaughter per day was about 15 to 25 swine in Addis Ababa abattoir and about 5 to 10 swine in Bishoftu abattoir. Slaughtering of swine was done once in a week, commonly, on Monday. (Figure 1).
Study design and sample size
A cross-sectional study was employed for the current study. Previous studies on the abattoir gross lesions prevalence of swine TB were reported to be 3.5% (34/984) 9 and 5.8% (49/841)10 in central Ethiopia. However, there was no a study report based on histopathological diagnostic techniques. Therefore, for the current study, the required sample size were calculated by considering 50% expected prevalence (p), 95% confidence interval (z = 1.96) and 5% desired absolute precision (d). The sample size was determined by making use of the formula after Thrusfield and Christley.23
Hence, ![]()
Where n = is the required sample size, p = is the expected prevalence, z = multiplier of the 95% confidence interval and d = desired absolute precision. By making use of the above formula the calculated sample size (n) was 384; however, to increase precision 556 swine were chosen from the two abattoirs.
Sample collection and sample processing
Non probability sampling method was employed to all slaughtered swine at Addis Ababa and Bishoftu abattoir. Hence, antemortem examination was carried out on all live swine. These included any abnormality in movement (gait), reaction to touch and sound, any visible discharges from natural orifices, coughing, lesions on body and body conditions. Data such as age, sex, origin and body condition scores were collected during antemortem inspection by discussion with the owners and abattoir swine care providers. Postmortem inspections were done and all collected tissues were cut in thin slices (roughly 3 mm wide), and the presence of macroscopic TBL was recorded 11 and Di Marco et al., 24 by observation of any visible gross lesions and by palpation of organs and lymph nodes during and/or after the animals were slaughtered. The lymph nodes inspected include submandibular, mesenteric, retropharyngeal, parotid, mediastinal, tracheobronchial lymph nodes as well as lungs, liver and spleen were inspected.
Pathology scoring was conducted on tissues with tuberculous lesions to determine the severity of the lesions. The lungs and lymph nodes were removed for the investigation of tuberculous lesions based on semi-quantitative procedure developed previously by Ameni et al.12 Briefly, lesions in the lobes of the lungs were scored separately as follows: 0 = no visible lesions; 1 = no gross lesions but lesions apparent on slicing of the lobe; 2 = fewer than five gross lesions; 3 = more than five gross lesions; 4 = gross coalescing lesions. The scores for the individual lobe were added to generate lung score. Similarly, the severity of gross lesions in individual lymph node was scored as follows: 0 = no gross lesions; 1 = small lesion at one focus; 2 = small lesions at more than one focus; 3 = extensive necrosis. Scores of individual lymph node were added and generated the lymph node score. The total pathology score per swine was obtained from the sum of lung and lymph node scores.
Gross lesions indicative of TB were observed on lung and/or lymph nodes to collect tissue samples. Briefly, approximately 2mm thick tissue specimens from active lesions were collected into sterile universal bottles filled with 5ml of 0.9% saline solution and transported on ice box packed with ice packs to keep the cold chain until they reached Aklilu Lama Institute of Pathobiology (ALIPB) TB laboratory for Mycobacterium culture. Whenever immediate culturing was not done then samples were kept at 4OC for two days and refrigerated at -200C when to stay for longer time to detect mycobacteria.13 Mycobacterial culturing was conducted under biocontainment safety cabinet level two (BSC-II) facilitated with HEPA filter (negative pressure). A presumptive diagnosis was made by histopathology by collecting tissue samples of 3-5mm in 10% buffered formalin from lung, liver, spleen and lymph nodes including active part of the lesion and some surrounding apparently normal tissue14 and taken to pathology laboratory of the National Animal Health Diagnosis and Investigation Center (NAHDIC).
Post-mortem and gross pathology
On the basis of gross pathology, in 556 slaughtered swine showed that 19.6% (109/556) were found to have tuberculous like lesions in parenchymatous organs of which 12% (69/556) were from lymph nodes, 5.7% (32/556) of the lungs, 1% (6/556) of the liver and 0.36% (2/556) of the spleen (Figure 2). The tuberculous like lesions found in lymph nodes, particularly those of mesenteric and retropharyngeal, were statistically significant (P < 0.05) than in lungs and associated lymph node. The majority of the lesions were considered to be of typical tuberculous lesions characterized by central round, oval, or irregular, often coalescing areas of caseous necrosis and calcification. The calcified lesions were exceedingly distributed in different lymph nodes and parts of the lungs (Figure 3).
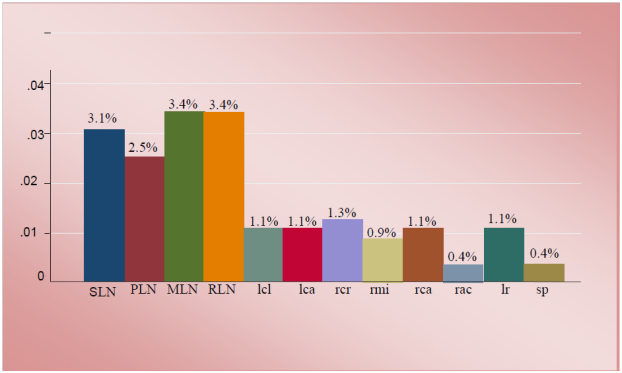
Figure 2 The mean prevalence of tuberculous like lesions in lymph nodes, lungs, liver and spleen. **SLN, submandibular lymph node; PLN, parotid lymph node; MLN, mesentric lymph node; RLN, retropharyngeal lymph node; LCR, left cranial; LCA, left caudal; RCR, right cranial; RML, right middle; RCA, right caudal; RAC, right accessory lobes of lungs; LR, liver; SP, spleen
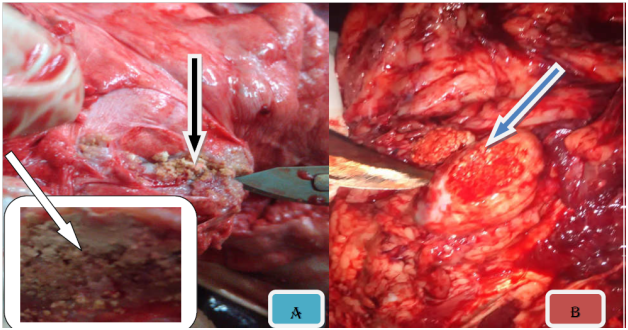
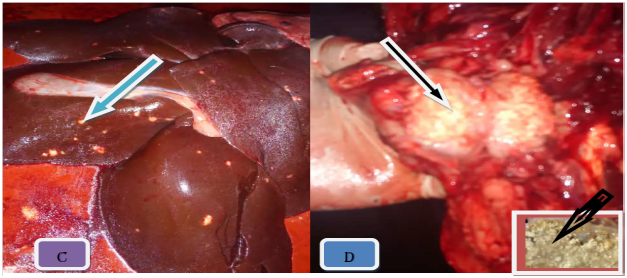
Figure 3 Gross TB like lesion in different organs. Note: The typical caseuos calcified on left caudal lung (A), caseous exudates lesions in retropharyngeal lymph node (B), multifocal hepatic nodules (C), and typical caseous exudates in submandibular lymph node; after parafinized (black arrow) (D).
Pathological lesions were commonly seen in mesenteric lymph node 3.4% (19/556) and retropharyngeal lymph node 3.4% (19/556) (Table 1). Among lung lobes found with TB like lesion right cranial, left caudal and left cranial lobes had 5 pathological lesion score with frequency 1.3% (7/556), 1.1% (6/556) and 1.1% (6/556) respectively (Table 2).
Based on the stages of granuloma, 4.7% (26/556) lesions were calcified granulomas, 4.1% (23/556) lesions were necrotized, 3.6% (20/556) lesions were pyogranulomatous and 2.5% (14/556) lesions were resulted granulomas without calcification (Table 3).
Lymph nodes |
Total pathological score |
Frequency |
Percent (%) |
SLN |
3 |
17 |
3.1 |
PLN |
2 |
14 |
2.5 |
MLN |
4 |
19 |
3.4 |
RLN |
4 |
19 |
3.4 |
Lr |
- |
6 |
1.1 |
Sp |
- |
2 |
0.4 |
* SLN, submandibular lymph node; PLN, parotid lymph node; MLN, mesentric lymph node; RLN, retropharyngeal lymph node; LR, liver; SP, spleen
Table 1 Frequency of lesions in lymph nodes, liver and spleen based on pathological score
Lobes of lung |
Total pathological score |
Frequency |
Percent (%) |
Lcr |
5 |
6 |
1.1 |
Lca |
5 |
6 |
1.1 |
Rcr |
4 |
7 |
1.3 |
Rml |
4 |
5 |
0.9 |
Rca |
5 |
6 |
1.1 |
Rac |
4 |
2 |
0.4 |
*LCR, left cranial; LCA, left caudal; RCR, right cranial; RML, right middle; RCA, right caudal; RAC, right accessory lobes of lungs
Table 2 Frequency of lesion in different lobes of the lung based on pathological score
Types of lesions |
Frequency |
Percent (%) |
CG |
26 |
4.7 |
N |
23 |
4.1 |
PG |
20 |
3.6 |
G |
14 |
2.5 |
*Note: CF, calcified granuloma; G, granulomas without calsification; N, necrotized lesions; PG, pyogranulomatous
Table 3 Frequency of different types of lesions in lymph nodes, lungs, liver and spleen
Association of different risk factors considered with gross lesions
The univariable and multivariable logistic regression analyses of the different putative risk factors considered for tuberculous like lesion positivity were indicated in Tables 4&5 respectively. Swine with poor body condition had higher odd (likelihood) to develop TB like lesions (OR=0.21, 95% CI, 0.11-0.38) than good body conditioned swine. Old aged swine were positive to have tuberculous like lesions (OR=3.14, 95% CI, 1.62-6.09) than younger ones.
Variables |
No. Swine |
No. Positive |
Prevalence of TB (%) |
OR |
χ2 |
P-value |
Sex |
||||||
Female |
297 |
43 |
14.47 |
0.1 |
0.75 |
|
Male |
259 |
40 |
15.44 |
1.07 |
||
BCS |
||||||
Poor |
264 |
21 |
7.9 |
26.81 |
0.000* |
|
Moderate |
174 |
28 |
16.09 |
0.47 |
0.010* |
|
Good |
118 |
34 |
28.81 |
0.21 |
0.000* |
|
Age (in year) |
||||||
1 |
254 |
26 |
10.23 |
27.58 |
||
2 |
212 |
28 |
13.2 |
1.33 |
0.319 |
|
3 |
72 |
19 |
26.38 |
3.14 |
0.001* |
|
4 |
18 |
10 |
55.56 |
10.96 |
|
0.000* |
BSC, body condition score; χ2, Chi-square; OR, odds ratio(crude); * = statistically significant
Table 4 Univariable logistic regression of different risk factors to postmortem lesion findings
Variables |
No. Swine |
χ2 |
OR |
95% CI for OR |
P-value |
|
BCS |
26.81 |
Lower |
Upper |
0.000* |
||
Poor |
264 |
|||||
Medium |
174 |
0.47 |
0.26 |
0.83 |
||
Good |
118 |
0.21 |
0.11 |
0.38 |
||
Age (in year) |
27.58 |
0.000* |
||||
1 |
254 |
1 |
||||
2 |
212 |
1.3 |
0.75 |
2.23 |
||
3 |
72 |
3.14 |
1.62 |
6.09 |
||
4 |
18 |
|
10.95 |
3.97 |
30.22 |
|
BSC = body condition score; CI = Confidence interval; χ2 = Chi-square; OR = odds ratio (adjusted); * = statistically significant
Table 5 Multivariate analysis of risk factors associated with postmortem lesion findings
Histopathological lesions
The most characteristic microscopic lesion was tubercle granuloma with central necrosis and calcification. The central area is made of necrotic cellular debris, calcium deposits, and connective tissue capsule walled off the granuloma from the surrounding tissue. The next layers from center to outer were made up of lymphycytes, macrophages, epitheloid macrophages distributed under connective tissue layers (Figure 4A). The frequency of characteristic tubercle granuloma was 4.7% (26/556) (Figure 4B). The presence of concomitant pyogranulomatous and granulomatous lesions in different organs was observed in 7.1% (40/556). Some granulomas were characterized by necrotic foci and intense calcification and fibrosis with absence of epithelioid cells. Multiple small granulomas in the lymph node (Figure 5) with less dense lymphocyte at periphery and epitheloid cells surrounding the deep outer lymphatic layer of the granuloma.
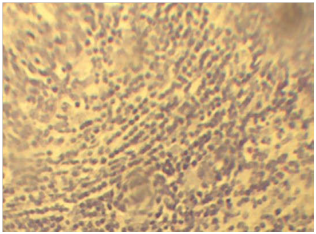
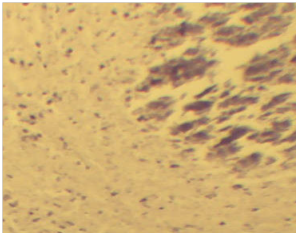
Figure 4 A) Characteristic epitheloid macrophages in the lymph nodes. Note; The epitheloid macrophages shown as elongated (Blue arrow) and the lymphocytes in the next layers (black arrows). H & E stains (10x). B) The characteristic granulomas in the lymph node with calcium deposits (red arrow) at the center, followed by sever necrosis and cellular debris (black arrow) immediate to calcium deposits. H & E stain (10x).
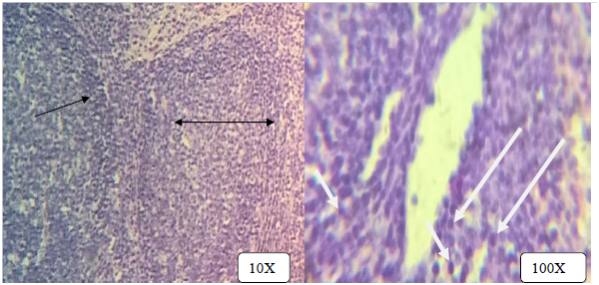
Figure 5 Multiple small granulomas in the lymph node with central necrosis, layer of dense lymphocyte at periphery (double headed black arrows) admixed with epitheloid cells (white arrow). H & E stains.
Multifocal hepatonecrosis with total removal of hepatocytes and infiltration of infilammatory cells (mixed mononuclear and polymorphoneuclear leukocytes) (Figure 6). At the periphery of this central necrosis are huge infiltrations of inflammatory cells (eosinophils, neutrophils and lymphocytes) in the portal triad region. Some foci of hepatic degeneration with swollen hepatocytes.
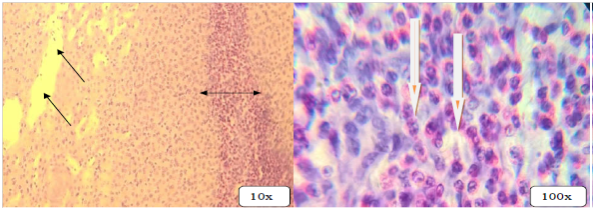
Figure 6 Multifocal hepatonecrosis with total removal of hepatocytes (black arrows), infiltration of inflammatory cells into portal traiads (double head arrows) and infiltration of inflammatory cells (white arrows). H & E stains.
Mycobacterial isolation and characterization
Mycobacterial culturing was conducted from characteristic tubercle lesions of which 7.5% (3/40) showed growth on primary culture media. Of those cultures which showed visible colony, 5% (2/40) were seen on Lowenstain-Jenson (L-J) media enriched with glycerol and the rest 2.5% (1/40) were grown on L-J media enriched with pyruvate. Three grown cultures were subjected to Zeihl-Neelsen staining technique in order to check the presence of acid fast bacilli. 5% (2/40) of the cultures were found to be acid fast positive. The observed colonial morphology was smooth whitish or yellowish color, sticky, off-white and breaks apart easily (Figure 7). The results attained of Z-N staining were cocci, short and some long rod shaped and also found in single and clumps (Figure 8), (Table 6).
Growth |
Z-N stain Positive (%) |
||||
Sample type |
L-J media with pyruvate |
L-J media with glycerol |
|||
Total |
Positive |
Total |
Positive |
||
SLN |
10 |
1(2.5) |
10 |
- |
- |
PLN |
7 |
- |
7 |
- |
- |
MLN |
10 |
- |
10 |
1(2.5) |
1(2.5) |
RLN |
8 |
- |
8 |
- |
- |
Lung |
5 |
- |
5 |
1(2.5) |
1(2.5) |
Total |
40 |
1(2.5) |
40 |
2(5) |
2(5) |
*SLN, sub-mandibular lymph node; PLN, parotid lymph node; MLN, mesenteric lymph node; RLN, retropharyngeal lymph node
Table 6 Bacterial culture result from tissues suspected tuberculosis lesions
There is little information on TB in swine and no report on histopathologic diagnosis with few published on swine TB in Ethiopia. The recognition of lesions of swine tuberculosis during meat inspection is of paramount importance for the surveillance and control of TB infection. Domingo et al.,27 indicated that failure to detect a lesion during abattoir meat inspection has the greatest significance in cattle with single lesion. As the same time; once the lesion is missed there is no further chance of detecting the disease in the animal. This idea also supports the chance of missing small sized lesions while inspecting swine carcasses at abattoir. Furthermore, the early slaughtering of swine before development of tuberculosis lesions in different organs and inadequate illumination in an inspection room of the abattoirs are the major factors determining the detection of tuberculous lesion/s. The slaughtered swine at an early age without sufficient time for lesion development and progression in the present study (45.6% (254/556)), was in agreement with the findings of the Domingo et al.27
The present study revealed tuberculous like lesions in 19.6% (n = 109) of swine carcasses inspected which was comparable to 19.8% reported by Cleaveland et al. 28 in cattle slaughtered in rural Tanzania. The current result was higher than 5.8% reported by Arega et al.,10 in Addis Ababa and Bishoftu abattoir in swine, 7.96% reported by Regassa29 in Wolaita abattoir in cattle, 8.8% reported by Biffa et al.,30 in Hawassa municipal abattoir in cattle, 9% reported by Nemomsa et al.,16 in Butajira abattoir in cattle and 11.50% reported by Abdurohaman31 in Butajira abattoir in cattle. On the other hand, the finding of this study was lower than 24.7% reported by Biffa et al.,30 and 24% by Mamo32 in cattle at Adama municipal abattoir. The higher prevalence recorded in the present study could be due to the fact that swine feed mainly poultry meal and their offal’s. Furthermore, the variations in prevalence could be attributed to the possible differences in the epidemiology of the disease in different species of animals, origin, housing, age, body condition scores of the animals and types of production system. The majority of swine under this study were originated from intensive poor management production system. According to Ameni et al.,15 Radostits et al.,42 and Mamo et al.,17 the intensive livestock management system could contribute to the development of mycobacterial infections than the extensive livestock management system which is in line with the current finding.
The difference in the prevalence of gross lesions between sexes was statistically insignificant (χ2= 0.1 and P > 0.05). The overall gross lesion prevalence in different organs of the both sex was 19.6% (109/556). This study result is higher than the previous studies by Teklu et al.,33 who reported 4.53% in cattle in Hossana, Shitaye et al.,9 who reported 3.5% in swine in Addis Ababa, Arega et al.,10 who reported 5.8% in swine in Addis Ababa, and to some extent consistent with the report by Cleaveland et al.,28 who reported 19.8% in cattle in Tanzania. The possible reason for this discrepancy might be due to the difference in lesions distribution of female and male animals, in which it was higher in females, sample size and study methodology. Less number of male swine and relatively more number of adult female swine as a means of culling from the breeding stock were presented for slaughter to the study abattoirs during the current study duration.
There was statistically significant difference in this study (χ2 = 27.58 and P < 0.05) between age and lesions in different organs (lungs, lymph node, spleen and liver). This result is in consistent with the reports of Gebremedhin et al., 34 who reported 2.6% in Dilla Municipal Abattoir in cattle and Nemomsa et al., 16 who reported 9% ( p < 0.05) in Butajira abattoir in cattle. The older swine (OR = 10.95) were nearly eleven times positive to have the gross pathological lesions than the younger ones. The results of the current finding also agreed with the findings of Barwinnek and Taylor,35 Ameni et al.,15 Regassa et al.,36 and Biffa et al., 37 in cattle who indicated as the age of the animal increases the probability of acquiring TB infection also increases. O’Reilly et al.,38 pointed out that; the protective capability is declining in aging animals due to a weaker immune system. Furthermore, Humblet et al., 39 indicated stresses, malnutrition and immunosuppression increase with age. The findings of all these authors supported the current result. The possible reason of difference between ages in current study will be the number of animals considered in each category is not proportional.
The effect of difference in the prevalence of swine TB among swine having different body condition scores was statistically significant (χ2 = 26.81; P < 0.05). The prevalence was highest in swine with poor body condition (47%) as compared to swine with medium body condition (21%) and good (8%) body conditions. This finding is in agreement with study reported by Nemomsa et al., 16 who reported the higher prevalence of tuberculosis lesions in poor body condition than good body condition animals (χ2 = 10.38; p < 0.05) in Butajira abattoir in cattle. This could be due to the weak protective immune response in poor body conditioned swine when compared to swine with good body condition. Moreover, TB in poor body conditioned swine may result in extensive lesions and wasting of the body due to its chronic nature. The present result is in consistent with previous reports of Collins et al.,40 Radostits et al.,41 and Radostits et al.,42 in those animals with good body condition have relatively good immunological response to the infectious agent than animals with medium and poor body condition scores. On the other hand, the animals with poor body conditions and in nutritional deficiency have reduced resistance to TB which is also in consistence with the report by Doherty et al. 43
A significant difference between the lungs and lymph node (P < 0.05) with lesions was associated with the route of infection. In addition, observation of tuberculous lesions in 3.4% (19/556) of the mesenteric and retropharyngeal lymph nodes may also imply that, the occurrence of intestinal tuberculosis was found to be more frequent than respiratory form. This finding is in agreement with the report described by Shitaye et al.,9 who observed tuberculous lesions in 33% (5/15) in mesenteric lymph node of swine that dictated that the occurrence of intestinal tuberculosis is more frequent than respiratory tubercolosis. However, the current finding is in disagreement with Corner [44], Neill et al.,45 Collins,46 Whipple et al.,47 and Teklu et al.,33 who reported that greater than 84% TB lesions due to M. bovis occurred in the respiratory system indicating inhalation/aerosolization in cattle to be the predominant route of transmission. Thoen et al.,1 described on the similar that ingestion of contaminated poultry faeces and offals and shared grazing with infected animals and/or ingestion of contaminated dairy products are the major sources of TB infection in swine.
Granuloma with calcification was the lesion most frequently detected in the examined organs and occurred in 4.7% of the cases (Figure 5) in the current histopathological finding. In consistence to this finding, Shitaye et al.,9 reported 3.4 % granulomatous lesions typical to swine TB. Granulomatous lesions typical to swine tuberculosis, manifesting granulomas with central necrosis surrounded by epitheloid cells distributed under connective tissue layers, were observed in 7.1%. Consistent to this finding, Whipple et al.,47 described that manifestation of typical granulomatous lesions in tissues with gross lesions was evident histologically.
The growth rate of mycobacteria on culture media in the current study was 7.5% (3/40). Amanfu48 and Cleaveland et al.,28 pointed out the poor growth rate of M. bovis on standard L-J medium. However, Teklu et al.,33 explained M. avium and M. tuberculosis to be well grown on standard L-J medium. Furthermore, Teklu et al.,33 indicated the presence of caseous and/or calcified lesions may not be truly tuberculous lesions; Pritchard 49 and Diguimbaye Djaibe et al.,50 also implied that viable mycobacteria may not be present in calcified lesions. The 7.5% culture positivity from the lesion current finding, was lower than 47% reported by Ameni et al., 51 35% reported by Müller et al.,52 32% reported by Shimeles,53 31.4% reported by Woyessa et al.,54 and 23.6% reported by Araujo et al.,55 in slaughtered cattle, and 30.6% by Arega et al.,10 in swine. However, the present finding was higher than that of Aknaw et al.,56 who reported 2.9% in slaughtered cattle at Bishoftu abattoir. This findings justify that the types of culture media and weakly developed and/or trace number of tubercle bacilli in swine tissue may result in lower growth rate.
Mycobacteria in this study were detected by Z-N staining in culture positive samples. This finding was consistent with a previous report by Gracey.57 M. bovis are often low in bovine specimens and they can be visualized by Z-N only if a limited quantity (at least 5×104 mycobacteria/ml) of mycobacteria are present.58
In conclusion, the results of this study show that, histopathological analysis followed by microbacterial isolation and Z-N staining indicated that swine can serve as a source of infection of tuberculosis to swine, human and/or other animal species. However, comparison of cultural isolation of Mycobacterium to pathological lesions indicated culture positivity to be smaller than lesions. This is due to the fact that lesions may be caused by other infectious agents rather than Mycobacterium species. Therefore, the diagnosis of mycobacteriosis in swine on a herd basis is important and usually directed to detect lymph nodes on the first hand and lungs as the second option from swine at slaughter. These results should be considered to prevent misdiagnosis of TB based on gross lesions and to establish specific control measures against these pathogens in pigs and related domestic animal species.
Conceived and designed the experiments: JS, KD, TD. Performed the experiments: JS, KD. Analyzed the data: KD, TD, SL. Contributed reagents/ materials/analysis tools: GM, GA. Wrote the paper: JS, KD, TD. Revised the final version of the paper for publication: TD, KD, GM, JS.
The authors are grateful to Addis Ababa Abattoir Enterprise and Bishoftu Abattoir for their support during sample collection. We also thankful for TB laboratory staffs at Aklilu Lemma Institute of Pathobiology and Faculty of Veterinary Medicine of Addis Ababa University for their support during the laboratory work.
Ethical approval was obtained from Addis Ababa University Colege of Veterinary Medicine dated 14/11/2107 (VM/ERC/05/10/018, 03/01/2018), Bishoftu, Ethiopia.
The author declares there are no conflicts of interest.

©2019 shiferaw, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.