International Journal of
eISSN: 2573-2889


Research Article Volume 4 Issue 6
Department of Biochemistry, Adekunle Ajasin University, Nigeria
Correspondence: Oluwaseun Fapohunda, Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Nigeria, Tel +2347062998896
Received: September 24, 2019 | Published: November 4, 2019
Citation: Fapohunda O, Balogun O. Oral magnesium supplementation modulates hepatic and intestinal expression of some carbohydrate metabolizing genes in type 2 diabetic rats. Int J Mol Biol Open Access. 2019;4(6):189-194. DOI: 10.15406/ijmboa.2019.04.00119
Aim: This study was carried out to determine the effects of magnesium supplementation on Glucose transporter 4 (GLUT-4), Phosphofructokinase 1 (PFK-1) and Glucagon like-peptide 1 (GLP-1) in type 2 streptozotocin-nicotinamide induced diabetic rats.
Methods: Total of 24 Wister rats (n=6) were used for this study and the treatment was for 28 days. Group 1: Rats (Normal Control) orally given distilled water; Group 2: Metformin treated diabetic rats orally given 100 mg/kg body weight metformin; Group 3: Orally given 100 mg/kg body weight metformin with 1000 mg/kg body weight magnesium supplement; Group 4: Diabetic Untreated control rats given distilled water. Measured data was analyzed statistically using Analysis of Variance (ANOVA) and graphically by Graph prism 8.0.2. Tukey test was used as the Post hoc test and statistical significance was considered at p<0.05.
Results: The GLUT-4 expression of Group 3 were significantly (p<0.05) higher when compared with Group 4. However, Group 1 expression of GLUT-4 was significantly (p<0.05) high in comparison with the Diabetic Groups. Group 3 was significantly (p<0.05) higher when compared with Group 2 in the mRNA expression of PFK-1 at week 4. There was significant (p<0.05) increase in the expression of GLP-1 of the treated rats but the metformin-magnesium supplement treated group showed higher value when compared with Diabetic Untreated Group. Moreover, Group 3 induced a significant (p<0.05) increase in the expression of GLP-1 in comparison with Group 2 while the Normal control was significantly (p<0.05) higher than the Diabetic groups. This study demonstrates that magnesium may mediate effective metabolic control by stimulating the insulin sensitivity, and increased levels of GLUT-4, PFK-1 and GLP-1 in diabetic rats.
Conclusion: This suggests magnesium supplementation as an adjunct therapy with metformin, helps in improving GLUT-4, PFK-1 and GLP-1 expression.
Keywords: metformin, magnesium supplement, glucose transporter 4, phosphofructokinase 1, glucagon like-peptide 1
Abbreviations: DM, diabetes mellitus; IDDM, insulin-dependent diabetes mellitus; NIDDM, non-insulin dependent diabetes mellitus; GLP-1, glucagon like-peptide-1; ANOVA, analysis of variance
Diabetes mellitus (DM) is a common and prevalent metabolic disorder characterized by hyperglycemia and abnormal lipid and protein.1 It is one of the five leading causes death in the world affecting both developed and developing countries alike. It was projected that about 300 million people will have the disease by the year 2025.2 According to,3 the main indication of diabetes mellitus is the hyperglycemia in blood which is due to inappropriate pancreatic insulin secretion or low insulin-directed fostering of glucose by target cells. Diabetes mellitus is divided into several categories but the two major types are type 1 and type 2. Type 1 known as Insulin-dependent diabetes mellitus (IDDM) is autoimmune disorder caused by auto aggressive T-lymphocytes that infiltrate the pancreas and destroy insulin producing β-cells leading to hypoinsulinemia and thus hyperglycemia.4,5 Type 2 is known as non-insulin dependent diabetes mellitus (NIDDM). It is characterized by insulin resistance in insulin-targeting tissues, mainly the liver, skeletal muscle, and adipocytes.6
Treatment of DM takes multimodal therapeutic approach due to its multifactorial pathogenesis. The three main forms of treatment include: (i) diet and exercise, (ii) insulin replacement therapy and (iii) the use of oral hypoglycemic agents.7
There are many oral hypoglycemic agents for the management of type-2 diabetes currently, such as biguanides and sulfonylureas, but these synthetic agents are associated with certain adverse side effects (such as hypoglycemia, drug resistance, dropsy and weight gain) and even toxicity.1,2 In addition, these therapeutic measures have inadequate efficacy or significant mechanism-based adverse effect when used as a combined therapy. Hence, there is need for drug that has fewer side effects and elicit less hypersensitivity with high anti-diabetic regulations. Therefore, it becomes imperative to explore and discover alternative drugs and dietary supplement or therapeutic adjunct which are safer and more effective.
Magnesium is the fourth most abundant cation in the human body and the second most predominant intracellular cation after potassium.8 Being an indispensable element, it is involved in nearly all vital physiological processes (including glycolysis) essential for survival of living organisms. It was reported by9 that the link between diabetes mellitus and magnesium is well established. Many enzymes in the liver require Magnesium (Mg2+) for their activity10 including pacemaker enzymes in the glycolytic pathway (e.g. phosphofructokinase-1, PFK-1). Mg2+ deficiency occurs during diabetes induction11 this should concomitantly affect the level of PFK-1 in a diabetic subject because Mg2+ is an important cofactor for the catalytic activity of this enzyme. Hence, the expression of PFK-1 gene was investigated in this present study. It was also examined whether Mg2+ affects GLUT-4 mRNA gene expression.
Glucagon like-peptide-1 (GLP-1) is produced through posttranslational processing of proglucagon and acts as a regulator of various homeostatic events. GLP-1 is synthesized in small amounts in the pancreas. It induces insulin production in developing, and to a lesser extent in the adult intestinal epithelial cells. It was earlier suggested by12 that GLP-1 production when induced in intestinal epithelial cells could represent a new therapeutic approach to diabetes mellitus.
Materials; reagents
Citric acid, Sodium citrate, picric acid, Chloroform, Magnesium Sulfate (MgSO4, Sigma Aldrich, Hamburg, Germany), Metformin (Merck pharma care spoxil), Nicotinamide (NAD), Streptozotocin (STZ, Sigma Aldrich, Hamburg, Germany), Sanitizer, Sucrose (Dangote PLC), Master mix (Biolabs, New England), Trizol (Lysis Solution Zymo Research, USA), Citric buffer solution, Agarose gel powder(Cleaver Scientific LTD-AG-100), Primers(Inqaba Biotechnical industries (pty) LTD, South Africa), Nuclease-free water (Life Science advanced technologies, England), Reverse transcriptase (Biolabs, New England), Random Primer (Biolabs, New England), Ethanol and Deoxynucleotide (dNTPs) solution mix (Biolabs, New England), Ethidium bromide, TBE buffer solution (Trisborate EDTA). All chemicals used were of analytical grade.
Animals
Twenty-four (24) male wister rats (average weight of 150 g) were obtained from the Department of Plant Science and Biotechnology, Adekunle Ajasin University Ondo State, Nigeria. They were housed under standardized environmental conditions (well-ventilated room, with 12 hour light-dark cycles and 55 ± 4 % at 24 ± 2°C). Standard pellet chow and water were given ad libitum.
They were divided into groups (n=6) based on their weight which was used to calculate the dosage of STZ, NAD, MgSO4 and metformin administered. Induction was carried out after three weeks of acclimatization. Administration started when the Wister rats were confirmed diabetic after 72 hours of induction. The intervention was carried out daily in the following order for four (4) weeks:
Group 1: Normal control rats given distilled water.
Group 2: Metformin treated rats were given 100 mg/kg body weight.
Group 3: Metformin + Magnesium treated rats were given 100 mg/kg and 1000 mg/kg body weight respectively.
Group 4: Diabetes untreated rats given distilled water.
Methods
Induction of diabetes in rats
NIDDM was induced as described by13 with little modification. Briefly, overnight-fasted rats were given intraperitoneal injection (i.p.) of freshly prepared 60 mg/kg STZ (dissolved in a citrate buffer of pH 4.8), 5 minutes after the i.p. administration of 110 mg/kg of freshly prepared NAD dissolved in normal saline.
Intervention
Magnesium sulfate and metformin were intubated into the mouth of the diabetic rats in quantity based on their body weights.
Sacrificing of animals and collection of tissue
At the end of 28 days’ treatment, the animals were subjected to fasting overnight and were sacrificed after 9 hours following ethical protocol. All experimental rats in each group were subjected to cervical dislocation following the ethical care and handling of experimental animals’ regularities and they were dissected using Dissecting set. The rats were sacrificed and tissues of interest (liver and intestinal crypt) were excised from each experimental animal. Little quantity of the excised tissues was dropped in eppendorf tubes containing 0.2 µml TRIzol across the groups and spin using laboratory centrifuge.
Gene expression profiling
RNA extraction
50μl of TRIzol® Reagent was added to the tissue sample. Sample was homogenized using a glass Teflon® or power homogenizer. Homogenized sample was incubated for 5 minutes at room temperature to permit complete dissociation of the nucleoprotein complex. 100μl of equal volume of chloroform per 50μl of TRIzol® Reagent was used for homogenization. The tube containing the samples was vigorously shaken by hand for 15 seconds. The samples were incubated for 2–3 minutes at room temperature. The samples were centrifuged at 4000 rpm for 30 minutes at 4°C. The aqueous phase of the sample was removed by angling the tube at 45° and pipetting the solution out. The aqueous phase removed was placed into a new tube and proceeded to the RNA Isolation Procedure. 100μl of 100% isopropanol was added to the aqueous phase, per 1 ml of TRIzol® Reagent used for homogenization. The samples were incubated at room temperature for 10 minutes and centrifuge at 4000 rpm for 30 minutes at 4°C. The supernatant was discarded from the tube, leaving only the RNA pellet. The pellet was washed with 100μl of 70% ethanol per 50μl of TRIzol® Reagent used in the initial homogenization. The samples were “vortexed” briefly and then centrifuged at 4000 rpm for 5 minutes at 4°C. The ethanol used for washing was discarded. The RNA pellet was air dried for 5–10 minutes. RNA was re-suspended in 50μl of sterile, nuclease-free water.
Complimentary DNA (cDNA) synthesis
By adding 2μl reverse transcriptase from the cocktail containing (1) the random oligo primer (2) the dNTP's (3) the reverse transcriptase and (4) the reverse transcriptase buffer (5) nuclease-free water, reverse transcription was performed. 2μl reverse transcriptase was aliquot into 20μl of total RNA across the samples for the conversion into cDNA. The sample is then incubated in the thermocycler running for 1 hour at 65°C in a total reaction volume of 22μl for the conversion.
Quantitative real-time polymerase chain reaction (PCR)
The amplification was performed using an optimization. Template (cDNA) 2μl, nuclease-free water 4μl, forward primers 1μl and reverse primers 1μl and master mix 2μl. All these reagents follow each sample for complete enzymatic reaction. The PCR was carried out using multigen optimax PCR machine (Serial Number: 1405010). Amplification conditions were: 94°C pre-denaturation for 5 min, denaturation at 94°C for 30 seconds, Annealing 55°C (Tm) for 30 seconds and Extension 72°C for 30 seconds and then 5 min at 72°C by 35 cycles. Expression levels of PFK-1, GLUT-4 and GLP-1 were normalized by Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequence of specific primer sets for each gene, amplicon sizes and annealing temperature of the primers real-time PCR time was used (Table 1).
Target gene |
Forward 5'-3' |
Reverse 3'-5' |
GAPDH |
TGA AGG TCG GAG TCA ACG GAT TTG GT |
CAT GTG GGC CAT GAG GTC CAC CAC |
GLUT 4 |
GCA ACA TGT CAG AAG ACA AGA TCA |
TAG CTC TTC GGT CAT CCA GAG |
PFK-1 |
CTG CAT CGG ACT CTA CCA GG |
AGG GAA AGG CAG TGG GTC TA |
GLP-1 |
ATC AAA GAC GCT GCC CTC AA |
CCA CGC AGT ATT GCA TGA GC |
Table 1 List of PCR Primer
Gel electrophoresis
Assessment of Polymerase Chain Reaction products (amplicons) were electrophoresed in 0.5% of agarose gel using 0.5x TBE buffer (2.6 g of Tris base, 5.4 g of Tris boric acid and 2 ml of 0.5M EDTA and adjusted to pH 8.2 with the sodium hydroxide pellet). 0.5 g of agarose powder was weighed and added to 50 ml of 0.5x TBE buffer and was put in the microwave to heat for 45 seconds until agarose dissolved. 0.2μl of ethidium bromide was added to aid visualization and 2μl of tracking dye was added to each sample (amplified gene). The agarose was poured into the gel tray with the combs and allowed to stay for 15 minutes for agarose to solidify; the gel box was filled with buffer. The combs were carefully removed, then 8μl of the samples were carefully loaded into the wells and the gel was run for 15 minutes at 100 V. The expression products were visualized as bands by UV-transilluminator (foto/photophoresis, Serial Number: FPG1 02046277).
Data and statistical analysis
Data are expressed as Mean ± standard error of mean (SEM). Statistical analyses were carried out using one-way Analysis of variance (ANOVA), with graphical representation done using Graph prism 8.0.2. (Graph pad software, San Diego California, USA). Tukey test was used as the post-hoc test and statistical significance was considered at 95% confidence interval (p<0.05).
Diabetes Mellitus (DM) is an endocrinological disorder and not a single disorder but a group of metabolic or heterogeneous afflictions resulting from an irregularity in insulin secretions, insulin actions or both.14,15 The failure of pancreatic beta cells to produce insulin, and the impairment of insulin action, play a central role in the disruption of glycemic homeostasis, leading to hyperglycemia, a hallmark of DM.16 At present, optimized glucose control is recognized as the best approach to reduce the risk of diabetes chronic complications.17 The present study was designed to investigate the effect of oral magnesium supplementation as adjunct therapy with metformin on PFK-1, GLUT-4 and GLP-1 genes.
Glucose transporter controls the rate of glucose utilization. In hepatic tissue there are three glucose transporters: Glucose transporter 1, 3 and 4 (GLUT-1, GLUT-3 and GLUT-4). Insulin increases uptake rate of glucose mainly by stimulating the translocation of the GLUT-4 isoforms from intracellular pools to the surface cell membrane by increasing rate of glucose transport.18
This present study showed that there was significant (p<0.05) difference in the expression of GLUT-4 gene in the diabetic groups when compared with the Normal Control group. This is an indicator of defective expression of GLUT-4 in diabetic state which is one of the hallmarks of type 2 diabetes. This result is in tandem with the findings of,18 who reported that there was down-regulation of GLUT-4 in diabetic subjects. All the Diabetic groups showed reduced level of GLUT-4 gene expression compared to the Normal Control as seen in Figure 1. Comparison was carried out between the treated and the untreated groups and the expression of GLUT-4 was significantly (p<0.05) higher in the treated groups than the diabetic untreated group. However, there was a significant (p<0.05) increase in the expression of GLUT-4 in the group administered with MgSO4 (1000 mg/kg) and metformin (100 mg/kg) compared to the group treated with Metformin only, suggesting that Mg mediates effective metabolic control by autophosphorylation and stimulation of insulin which elicit the translocation of GLUT-4 from an intracellular pool to the plasma membrane thereby facilitating glucose transport and lowering blood glucose level according to.19 Hence, it appears that the modulated expression of GLUT-4 in insulin target hepatic tissues influences the regulation of glucose uptake and metabolism as well as reflecting the situation of the whole-body insulin resistance, which is a major factor in the pathogenesis of T2DM.
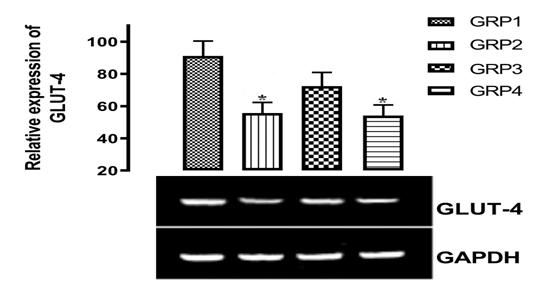
Figure 1 Effect of magnesium supplementation in the expression of glut-4 in the hepatic tissue. (*) means significant difference p<0.05 when compared with normal control (GRP1).
PFK-1 is an allosteric enzyme and it is critical for the metabolic regulation of the glycolytic pathway. Although, phosphorylase responds to an extra-cellular stimulus, PFK-1 is sensitive to the intracellular level of several allosteric effectors. Pi, ADP, AMP and fructose 2, 6-bisphosphate are positive effectors, which stimulate PFK-1 activity that is, increases the level of fructose 2,6-bisphosphate in the cell.20 The stimulation of these PKF-1 enzymes by insulin in the hepatic tissues which leads to enhanced glycolysis, is of fundamental metabolic importance for the following reasons: (1) when glycogen store in liver is replete, the glucose is converted to lactate, to maintain enhanced glucose utilization and (2) lactate produced and released by muscle and adipose tissue is taken up by liver and converted to glycogen.21 Moreover, the mRNA expression of PFK-1 at pH 8 was significantly (p<0.05) increased with 90 µg/dl and 100 µg/dl in GRP2 and GRP3 respectively but with a significant(p<0.05) decrease of 60 µg/dl in untreated diabetic rats.
Wister rats in GRP4 (Diabetes Untreated) showed significant (p<0.05) reduction in the level of PFK-1 expression when compared with the Normal Control (GRP1) rats in Figure 2. The graphical illustration in Figure 2 shows that rats in GRP4 were completely diabetic with shattered glycolytic pathway which cannot produce pyruvate that will go through TCA cycle. Hence, there will be reduced energy, body and muscle weakness. The treated and the untreated groups were also compared and the expression of PFK-1 was significantly (p<0.05) higher in the treated groups than the Diabetic Untreated Group. There was a significant (p<0.05) difference in the level of PFK-1 expression in the group administered with MgSO4 (1000 mg/kg body weight) and metformin (100 mg/kg body weight) compared to the Metformin treated Group. Most of enzyme(s) in the Glycolytic pathway are kinases or phosphorylase which requires Mg2+ as their cofactor. Increase in intracellular Mg helps to keep the enzyme in their activated form to continue the process of glucose conversion to pyruvate that will help to synthesize more ATP through Citric acid pathway and reduce risk of hyperglycemia. Activating protein kinase (AMPK) system is a key player in regulating energy balance at both the cellular and whole body level. Liver AMPK controls glucose homeostasis mainly through the inhibition of gluconeogenic gene expression and hepatic glucose production. Mg helps to activates AMPK in a calcium-dependent manner which in turn activates PFK-1 in the glycolytic pathway.22
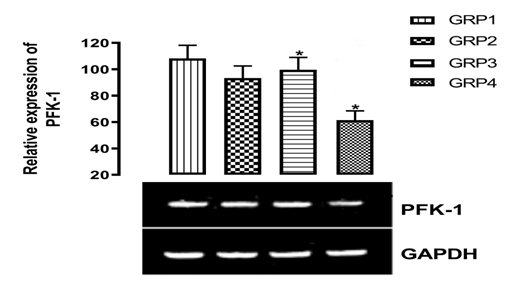
Figure 2 Effects of magnesium supplementation on phosphofructokinase (pfk-1) expression in the hepatic tissue. (*) means significant difference p<0.05 when compared with normal control (GRP1).
The up regulation of GLP-1 increases the level of the hormones called 'incretins'. These hormones help the body to produce more insulin when needed and reduce the amount of glucose produced in the liver. Metformin induced a significant (p<0.05) increase in the expression of GLP-1 in GRP2 compared to GRP4 (Diabetic Untreated) as observed in Figure 3. This is consistent with the findings of23 who reported that in pooled rat model, metformin significantly inhibits degradation of GLP-1 (7-36) amide after 4 weeks of administration.

Figure 3 Regulatory effect of magnesium supplementation on the expression of glucagon like-peptide (glp-1) in the intestinal crypt. Asterisk (*) means significant difference p<0.05 when compared with normal control (GRP1).
Significant (p<0.05) increase of GLP-1 mRNA expression levels were observed in GRP2 and GRP3. These up regulation of GLP-1 stimulates insulin secretion and reduce glucagon secretion in a glucose-dependent manner, improve satiety and promote weight loss.24,25 According to,26 increase in GLP-1 expression has been shown to improve cardiovascular outcomes and have high glucose lowering effect. The modulation and up-regulation of GLP-1 must have resulted from the inhibition of a gastrointestinal enzyme Dipeptidyl peptidase IV (DPP-4), because DPP-4 is an enzyme that degrades incretins.
This study acknowledges the technical support of the Director of center for Bioinformatics and Drug Design (CBDD) Adekunle Ajasin University, Dr. I.O. Omotuyi and Inyang Kayode for his assistance with the molecular assays.
No funding source supported this study.
The authors declares there is no conflicts of interest.

©2019 Fapohunda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.