International Journal of
eISSN: 2573-2889


Research Article Volume 5 Issue 1
CEO & Founder of Biomix Network INC. USA, India
Correspondence: Pawan Saharan, CEO& Founder of Biomix Network INC. USA, India
Received: March 06, 2020 | Published: March 31, 2020
Citation: Saharan P. Meta-analysis of interventional/prospective phase III (1Year duration) accelerated studies to determine the efficacy & safety of RECEPTOL® liquid spray used as a stand- alone mono therapy in HIV/AIDS patients with multiple symptoms. Int J Mol Biol Open Access. 2020;5(1):28-31. DOI: 10.15406/ijmboa.2020.05.00127
Creating a Paradigm Shift from Treatment to Prevention of Communicable Disease in India with over 10 years follow up of HIV Patients that could be an Answer to Covid-19 Virus, Swine flu & SARS?
A new corona virus, declared as Global Health Emergency (PHEIC) by WHO, 2019-nCoV was first identified in Wuhan, the capital of China's Hubei province, after people developed SARS like pneumonia. The incubation period (time from exposure to the development of symptoms) of the virus is 2-10 days and can be contagious during this time. Symptoms include fever, coughing, and breathing difficulties. Without a clear cause of 2019-nCoV, treatments with existing vaccines is not effective.1–6
1st PHEIC was declared by WHO for Swine Flue (H1N1 Influenzas) in 2009 with 284,500 death with 763 million cases reported in 214 countries and was successfully contained by Receptol® used at 3 domestic & international airports in Mumbai by Mumbai International Airports Ltd.7–15 The Patented Receptol® oral spray (USA Patent # US 9,249,188 B2) is a new Immunity Drug (NID) providing mode of action in a vaccine like manner with active immunity, can be used fortreatment and as preventative vaccine in dealing with current 2019-nCoV epidemic.
Receptol® consists of cell to cell communicator Nano informational peptides (Radha108) & proline-Rich Polypeptides (PRPs) from colostrum, Mother’s 1st milk after the birth of the child or calf.
Numerous Studies have shown that Receptol® spray will deliver nanopeptide crossing blood brain barrier and have great effectiveness in treating many immunity disease including all viral infections.15–20 As a natural product, Radha-108 Nanopeptides have no side effects which can be taken safely by all age and are not species specific.
Receptol® showed great effectiveness in treatment of retrovirus such as HIV, Swine Flu and SARS like conditions caused by Coronavirus. An accelerated, prospective Phase III global efficacy and safety studies for Receptol® (containing API of Radha 108 Nanopeptides) was conducted for HIV Positive patients with 10 years follow up showed significant resolution of all symptoms and pharmacological effects with low to NILL Viral Load.21–26
Once RADHA108 series get absorbed in the blood stream through buccal mucosa or transdermal route and crosses the Blood Brain Barrier (BBB), they act on Pituitary gland in brain and cell to cell communicator informational proteins (RADHA108) in RECEPTOL® will active in mitigating cell fusion. RADHA108 series has shown to dock on glycoprotein receptor on the cell surface and thus closing doors and windows for viral entry into the cell surface & immune cells in particular.27–28
Coronaviruses is enveloped positive- RNA viruses characterized by club-like spikes that project from their surface. It has unusually large RNA genome, four main structural proteins. spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins as virus particles. S protein is cleaved by a host cellfurin-like protease into two separate polypeptides noted S1 and S2. The initial attachment of the virion to the host cell is initiated by interactions between the S protein and its receptor.
The S-protein/receptor interaction is the primary determinant for a coronavirus to infect a host species and also governs the tissue tropism of the virus. Receptol® nano-peptides can block the attachment of S protein like In case of well-studied mode of action in treatment of retrovirus infection such as HIV.
Receptol® oral spray invented via Dr Pawan Saharan, Founder, Biomix USA, Australia and INDIA ( www.biomix.in) can be an answer to prevent & treat current epidemic of 2019-nCoV as its Mode of Action is similar to that of AIDS for which global studies with 10 years follow is done.
Receptol®’s RADHA108 series nano-peptide will not only dock on S glycoprotein receptor on the cell surface to mitigate cell fusion closing doors and windows for all viral entry but will also stimulate the maturation of immature thymocytes into either helper or suppressor T cells, T help cells will help produce antibodies against 2019nCoA, Suppressor T cells, on the other hand, deactivate other lymphocytes after an infection has been cleared to avoid damage to healthy tissues. Receptol® will help to produce memory T cells, in order to expedite the production of antibodies for future infection in all human hosts.29–30
Primary objective
To evaluate the efficacy of RECEPTOL® liquid in HIV/AIDS patients in terms of reduction in HIV viral load and HIV/AIDS related clinical symptoms.
Secondary objective
To determine the effect of oral spray administration of RECEPTOL® liquid on:
Overall Assessment of Efficacy and Safety/Tolerability of RECEPTOL®.
Study plan
Above Table 1 reveals that mean CD4 count was 265.95 at baseline. Table 2.
|
|
Mean CD4 count |
|
Duration (months) |
( X! SD) (N = 60) |
|
Baseline |
265.95 ± 151.63 |
|
1 |
310.33 ± 151.60 |
|
2 |
371.40 ± 158.38 |
|
3 |
428.55 ± 163.92 |
|
4 |
479.55 ± 172.81 |
|
5 |
541.48 ± 192.57 |
|
6 |
588.70 ± 196.49 |
|
7 |
641.28 ± 212.33 |
|
8 |
691.15 ± 226.13 |
|
9 |
742.28 ± 243.50 |
|
10 |
796.58 ± 257.41 |
|
11 |
859.33 ± 272.31 |
|
12 |
955.58 ± 298.27 |
Table 1 Profile of mean cd4 counts among study cases
|
Duration (months) |
Mean CD4 counts ( X! SD) (N = 60) |
|
Diff (Baseline – 1 ) (P value) |
*044.38 ± 041.09 (0.001) |
|
Diff (Baseline – 2 ) (P value) |
*105.45 ± 080.47 (0.001) |
|
Diff (Baseline – 3 ) (P value) |
*162.60 ± 106.78 (0.001) |
|
Diff (Baseline – 4 ) (P value) |
*213.60 ± 128.38 (0.001) |
|
Diff (Baseline – 5 ) (P value) |
*275.53 ± 162.29 (0.001) |
|
Diff (Baseline – 6 ) (P value) |
*322.75 ± 177.10 (0.001) |
|
Diff (Baseline – 7 ) (P value) |
*375.33 ± 200.20 (0.001) |
|
Diff (Baseline – 8 ) (P value) |
*425.20 ± 219.85 (0.001) |
|
Diff (Baseline – 9 ) (P value) |
*476.33 ± 241.64 (0.001) |
|
Diff (Baseline – 10 ) (P value) |
*530.63 ± 260.67 (0.001) |
|
Diff (Baseline – 11 ) (P value) |
*593.38 ± 278.82 (0.001) |
|
Diff (Baseline – 12 ) (P value) |
*689.63 ± 311.98 (0.001) |
|
By ANOVA *Significant |
Table 2 Changes in mean cd4 counts among study cases
By ANOVA *Significant
After 1 month of treatment, mean CD4 count showed a significant rise of 16.7% from baseline.
Same trend was observed at the end of 12 months of treatment.
According to this data, mean weight was 53.13 kg at baseline.
After 1 month of treatment, mean Weight showed a significant rise of 1.2% from baseline. Tables 3&4.
|
Duration (months) |
Mean Weight (kg) ( X! SD) (N = 60) |
|
Baseline |
53.13 ± 9.55 |
|
1 |
53.75 ± 9.51 |
|
2 |
54.47 ± 9.50 |
|
3 |
55.28 ± 9.54 |
|
4 |
56.28 ± 9.26 |
|
5 |
56.66 ± 9.57 |
|
6 |
57.37 ± 9.59 |
|
7 |
58.10 ± 9.75 |
|
8 |
58.79 ± 9.70 |
|
9 |
59.58 ± 9.82 |
|
10 |
60.27 ± 9.73 |
|
11 |
61.11 ± 9.83 |
|
12 |
62.11 ± 9.75 |
Table 3 Profile of mean weight among study cases
|
Duration (months) |
Mean Weight (kg) ( X! SD) (N = 60) |
|
Diff (Baseline – 1 ) (P value) |
0.62 ± 0.48 |
|
Diff (Baseline – 2 ) (P value) |
1.34 ± 0.75 |
|
Diff (Baseline – 3 ) (P value) |
2.15 ± 0.94 |
|
Diff (Baseline – 4 ) (P value) |
2.83 ± 1.16 |
|
Diff (Baseline – 5 ) (P value) |
3.53 ± 1.37 |
|
Diff (Baseline – 6 ) (P value) |
4.24 ± 1.58 |
|
Diff (Baseline – 7 ) (P value) |
4.97 ± 1.77 |
|
Diff (Baseline – 8 ) (P value) |
5.66 ± 1.99 |
|
Diff (Baseline – 9 ) (P value) |
6.45 ± 2.12 |
|
Diff (Baseline – 10 ) (P value) |
7.13 ± 2.19 |
|
Diff (Baseline – 11 ) (P value) |
7.97 ± 2.48 |
|
Diff (Baseline – 12 ) (P value) |
8.97 ± 2.81 |
Table 4 Changes in mean weight among study cases
By ANOVA *Significant
Figure 1 Same trend was observed at the end of 12 months of treatment.
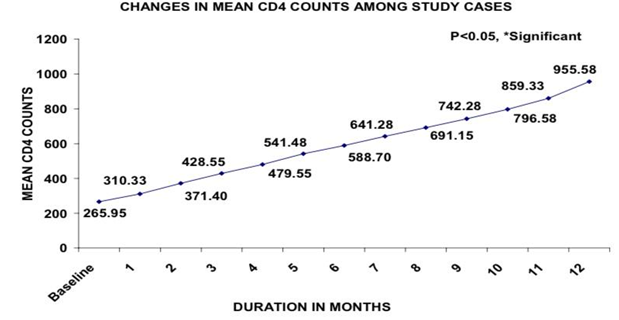
Figure 1 As per this figure, mean CD4 count significantly increased after treatment of Receptol liquid spray from 1 month onwards till end of treatment from baseline.
The below Figure 2 indicates that mean weight significantly increased after treatment of Receptol liquid spray from 1 month onwards till end of treatment from baseline.
The results of this an interventional / Prospective study reveals that after RECEPTOL Oral Spray therapy, average CD4 count and mean weight of study cases showed significant increase from baseline to end of treatment.
Hence we can conclude that, RECEPTOL is very effective and safe among HIV/AIDS cases to increase the weight and overall wellness that could be an Answer to Covid-19 Virus, Swine flu & SARS?
None.
The author declares there are no conflicts of interest.

©2020 Saharan. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.