International Journal of
eISSN: 2573-2889


Research Article Volume 7 Issue 1
1Laboratorio de Patología, Facultad de Odontología. Universidad Nacional de Tucumán, Argentina
2Centro de referencia para Lactobacilos (CERELA-CONICET), San Miguel de Tucumán, Argentina
3Facultad de Ciencias Naturales. Instituto Miguel Lillo. Universidad Nacional de Tucumán, Argentina
Correspondence: Alejandra de Moreno de LeBlanc, Centro de Referencia para Lactobacilos, Chacabuco 145 (4000). San Miguel de Tucumán, Tucumán, Argentina
Received: January 09, 2024 | Published: January 22, 2024
Citation: Carino S, De Moreno LA, Aybar OA, et al. Hyperplastic, dysplastic and neoplastic lesions associated with a model of carcinogenesis induced by DMBA in the salivary glands of BALB-c mice.Int J Mol Biol Open Access. 2024;7(1):8-13. DOI: 10.15406/ijmboa.2024.07.00158
The aim of this work was to develop a mouse model of submandibular salivary gland carcinogenesis by chemical induction and to perform a revision of the literature on similar models using the carcinogen 7,12-Dimethylbenz[a]anthracene (DMBA). The descriptive review was carried out on published works in English and Spanish languages, from the period 1977-2023, which included different animal models of chemical carcinogenesis induced by DMBA in salivary glands. Articles were reviewed in order to analyze the effectiveness of the carcinogenesis induction. For our model, BALB/c mice were used. Animals were anesthetized and after surgery to expose the submandibular gland, each mouse was injected with solution of DMBA 2% in corn oil. Mice were monitored weekly until sacrifice at week 12. The samples were processed with the routine histological methods. The results from the descriptive review showed that DMBA is mainly administered by Intraglandular injections into the submandibular glands after surgical exposure, being both pellets and solutions used for the induction, and rats more common than mice. This revision summarizes the research results of previous studies and demonstrates which model has greater reproducibility. Our results showed that one single injection of DMBA directly into the surgically exposed submandibular gland was an optimal technique to induce the tumor at the desired site. Edema was observed in the surgical area during the first 2 weeks. From week 5, the mice presented internal hardness and hair loss in the glandular area; 50% of the animals presented palpable and measurable tumor masses from week 10. Microscopic observations showed that 89% of the mice developed malignant neoplasms: Carcinomas in situ, micro-invasive and invasive (6/8); sarcomas (1/8), and carcinosarcoma (1/8). Associated lesions were atypical ductal hyperplasia; periductal fibrosis, and sarcomatous stroma. This model marks a difference with the subcutaneous injection technique, without surgery, which does not allow the carcinogen to be adequately directed to the gland.
Keywords: DMBA, salivary glands, cancer, experimental model, mice, histology
Salivary gland tumors are rare and constitute between 3 to 6% of head and neck tumors; the majority being benign. The most frequent occur in the parotid gland (70%), being less common in the minor salivary glands (20%), and in the submandibular glands (10%).1 The histopathology of salivary gland tumors presents great diversity, and the anatomopathological diagnosis is an area under constant study within head and neck pathologies. In this sense, the World Health Organization updated the classification of salivary gland tumors in the 5th edition of the Classification of Head and Neck Tumors, based on their molecular pathological characteristics.2 For this reason, it is very useful to have adequate models, both in vitro and in vivo, in order to analyze possible therapies.
Regarding submandibular salivary gland carcinogenesis, different approaches have been used over the years to have appropriate experimental models, including the use of viruses,3 radioactive isotopes and genetically modified animals;4 highlighting chemical induction as one of the most used methods to induce carcinogenesis in animals.5 7,12-dimethylbenz[a]-anthracene (DMBA) is a known carcinogen, used to induce a variety of tumors in rodents with high frequency.6 Chemically induced DMBA carcinogenesis in mouse skin is the most extended and analyzed, and these studies have led to a better understanding of the multiple stages of epithelial cancers.7 Mammary tumors can also be induced with single doses of DMBA in susceptible rat strains.8 In addition, DMBA was also used to induce tumors in salivary glands, for which different ways of or administration have been proven; among them the subcutaneous injection and the injection after gland exposure; being this last one the most specific.5–11
Hamsters, rats and mice, and different routes of DMBA administration have been proposed as animal models for chemically induced carcinogenesis in the salivary glands. The specificity of the appearance of tumors in the salivary glands is related to the route of administration. Rats and mice are the most common experimental animals used for the development of salivary gland tumors, especially due to their size; in addition, hamsters were described as a model in which the right buccal pouches were painted three times per week with DMBA. In this last model, the percentage of animals with tumors was close to 50% at fifteen weeks, and the systemic administration of the immunosuppressant dexamethasone reduced the time to tumor appearance, but the percentage of animals with tumors did not exceed 60%.12 In rats, the development of salivary gland tumors was studied after submandibular glands were exposed by a surgical procedure and a sponge was used as a carrier of the carcinogen. The sponge containing a solution of DMBA with acetone was implanted into the glandular tissue. The methodology showed specificity since all the induced tumors were squamous cell carcinomas (SCC) and were presented in the gland. It was not associated with metastasis.5 Greater efficacy was achieved by injecting a solution of DMBA dissolved in acetone directly into the submandibular glands. Each rat received six to seven injections. Histological examination revealed adenocarcinomas of the submandibular gland in 100% of the female rats but not in the male rats which mainly developed fibrosarcomas, although this sex difference could not be explained. Thus this model is mainly proposed for female Wistar rats.13 In another work, with male rats, DMBA was also injected into the submandibular glands. The authors highlight that this model allowed to investigate glandular carcinogenesis from the beginning with the inflammatory changes until the appearance of the tumors.14 Mice are one of the most used rodents for the development of experimental models, especially due to their size. Described models used DMBA pellet implants without vehicle or support,15 or solutions.9,11
The aim of this work was to develop and describe histopathologically a model of carcinogenesis of the submandibular salivary glands in mice by chemical induction with a single injection of DBMA. In addition, a descriptive review of the literature on similar models (in rats and mice) of DMBA-induced carcinogenesis in salivary glands was carried out with the purpose of synthesizing, analyzing and grouping the results of selected articles, with emphasis on comparing them with our model.
Descriptive review
Different experimental models of carcinogenesis induced with DMBA in salivary glands were analyzed in order to summarize the effectiveness. A literature search from 1977 to 2023 was performed in the following databases: PubMed, Web of Science, and Google Scholar. A total of 38 papers were selected. The search included articles in English and Spanish. The keywords used to search for articles on experimental models were: tumorigenesis and salivary glands and DMBA; carcinogenesis and DMBA and salivary glands; carcinogenesis and DMBA and salivary glands and immunohistochemically; carcinogenesis and salivary glands and DMBA; salivary glands tumours and DMBA; salivary glands tumors and DMBA; salivary glands and DMBA and ROS; salivary glands tumors and DMBA and mice; salivary glands tumors and DMBA and rats.
From each experimental work, the following was recorded: authors, year of publication; possible combinations used with DMBA; single or multiple doses; drug concentration, experimental animal, method of application; duration of the experiment and results (percentage of tumors induced, types of neoplasms, reproducibility, etc.). Finally, the results obtained were compared with our model of carcinogenesis in the submandibular gland of BALB/c mice; both induced with DMBA. This comparison was made considering the induced tumor types, presence of non-neoplastic lesions, pre-neoplastic evidence of tumor progression, and unwanted effects in the experimental model.
Development of the model of submandibular glands’ carcinogenesis induced with DMBA in mice
Six to seven-week-old BALB/c mice (20–30 g) were obtained from the animal facility of Centro de Referencia para Lactobacilos (CERELA-CONICET, San Miguel de Tucumán, Argentina), and were tagged and distributed in metal housing cages at constant room temperature (20±2ºC) and relative humidity (60%), with a 12-h light-dark cycle. Food and water were provided ad libitum. Animals were treated according to a protocol approved by the Animal Protection Committee of CERELA (CRL-BIOT-LI-2015/1A). Mice were anesthetized with a mixture of ketamine hydrochloride (Laboratorios König S.A. Laboratories, Buenos Aires, Argentina), 100µg / g body weight, and xylazine at 2% (Bayer, División Sanidad Animal, Buenos Aires, Argentina), 5µg/g body weight, and then underwent surgery to expose the submandibular gland. Each mouse was injected with 100µl of a 2% solution of 7,12-dimethyl-benzoanthracene (DMBA, Sigma, St. Louis, MO, USA) in corn oil (Figure 1) shows a schematic representation of the procedure performed). The mice were checked weekly and the characteristics of each animal were recorded, including modifications on the skin, changes in the general state of the animal (weight loss, bristly hair, etc.), and palpation of possible lesions. Sacrifice was performed 12 weeks post-injection. Mice were euthanized under anesthesia and the samples from submandibular glands were fixed in formaldehyde solution (10% v/v in PBS) for histological evaluation. Samples were processed for paraffin embedding using standard methods; sections of 4 µm were cut and stained with hematoxylin and eosin (H&E). Samples were observed under a light microscope (Carl Zeiss- Axio Scope.A1).
Models of DMBA-induced carcinogenesis in salivary glands described by using rats or mice
Twelve articles were analyzed (Table 1). Two different formulas of the carcinogen were used; 9,10-dimethyl 1,2-benzantracene was used in 6 articles (6/11) and 7,12-dimethylbenzantracene was used in 4 articles (4/11), while one of the articles does not specify the chemical form used (only mentions DMBA). The animals used were rats (6) and mice (5). Different techniques were used for the induction of salivary gland tumors. The most widely described was the intraglandular injection in the submandibular (8/11), which was performed by surgical exposure under anesthesia (7/8). Granules or pellets were used in 4 articles. When the carcinogen was administered as a solution, acetone was the diluent most frequently used (3/4); the remaining protocols diluted the carcinogen in mineral oil. Regarding the use of granules or pellets, it was carried out without a vehicle by using a short needle with a plunger inside. Regarding the dose, most of the protocols (7/8) used one single dose. In the form of pellets or granules, 1 mg of DMBA for mice and 5 mg of DMBA for rats have been reported. In the form of a solution in acetone, the concentrations varied between 0.5 and 2%, all in rats. The protocol that shows the most differences was described by Zaman,13 et al who performed an injury (excision of a small portion of tissue), then injected the carcinogen (1%) and equal amounts of the carcinogen were then injected biweekly, with each animal receiving six or seven injections. In that article, the injury performed was an influential factor in the development of adenocarcinomas. Actis,16 et al carried out a model in mice by induction with a subcutaneous injection in the submandibular area with DMBA dissolved in corn oil. Finally, another described protocol administered DMBA dissolved in sesame oil to rats by nasogastric tube, following weekly doses (65 mg/kg of body weight) for 6 weeks.17 The duration of the protocols was variable; in most of the articles, the percentage of animals that developed salivary gland cancer was greater after 5 weeks. The use of male animals was more frequent than female, both for rats and mice. In this sense, the work of Zaman, et al.13 Made a differentiation between females and males, demonstrating that the type of tumors developed were different; in female rats predominated adenocarcinomas in the submandibular glands (100%), some associated with fibrosarcomas. In contrast, male rats developed mainly fibrosarcomas (11/12); thus, this model was mainly proposed for female Wistar rats.
|
Animal model |
DMBA admisnitration |
Experimental Model |
Dose |
Total Duration |
Early injuries |
Tumor presence |
Reference |
|
Rat |
Granules |
Intraglandular injection after surgical exposure |
5 mg (1 single dose)
|
8 weeks |
Early onset and extensive squamous metaplasia and keratinization in the central area of the implan site (4 weeks). Inflammation, fibrosis, cystic degeneration. |
Tumors in 100% of the animals (8 w). Pronounced malignant change and necrosis in some metaplastic islands and infiltration adjacent glandular tissue. |
|
|
|
DMBA dissolved in acetone.
|
Intraglandular injection by surgical exposure |
1% in the exposed gland followed by biweekly injections (6 or 7 in total) |
20 weeks |
Not specified |
Tumors in 100% of the animals. Adenocarcinomas in all female rats, 50%x of which were associ-ated with fibrosarcomas. Male rats developed fibrosarcomas, and one of them squamous-cell carcinoma. |
Zaman et al.13 |
|
|
DMBA dissolved in acetone
|
Intraglandular injection by surgical exposure |
2% (1 single dose) |
20 weeks |
Enlargement of the gland after 10 weeks. Sialadenitis with acinar atrophy, dilatation of the ducts and a stroma composed by neutrophils. Hyperemia cases with stromal oedema, vascular proliferation and few inflammatory cells. of the neck after 20 weeks |
Percentage of tumor developed is not specified. Most animals developed squamous cell or carcinosarcoma. Sarcoma was also observed in some rats.
|
Mainenti et al.14 |
|
|
DMBA dissolved in acetone
|
Injection in submandibular gland after surgical exposure |
0.5% (1 single dose) |
150 days |
Epithelial proliferation in the glandular stroma (7d); epithelial cells arranged as cords and nests. Structures similar to ducts, some with epidermoid differentiation, leukocyte infiltration, hemorrhagic areas and fribous tissue (30 d). |
Carcinoma |
Brunotto et al.10 |
|
|
DMBA dissolved in sesame oil
|
Gastric gavage |
65 mg/kg of body weight (weekly dose for 6 weeks) |
|
Inflammation, degeneration and/ or allergic reactions. Dysplasia, especially in the serous acini. Sialadenitis, inflamation, mast cell infiltration, and apoptosis in ductal system
|
Not specified types or percentage of tumors |
Abdul Khalik et al.17 |
|
Mouse |
granules |
Intraglandular injection after surgical exposure |
1 mg (1 single dose) |
15 weeks |
Ductal proliferation, squamous metaplasia of ductal epithelium, epidermoid cyst with prominent keratin formation. |
Tumors in 91% of the animals (11w). Squamous cell carcinomas (75%) appeared early than sarcomas (20%). Adenoma was observed in one mouse
|
El-Asfuhani et al.20 |
|
|
granules |
Intraglandular injection after surgical exposure |
1 mg (1 single dose) |
7-14 weeks |
Not specified |
Epidermoid carcinoma in 100% of mice (20), two of them developed squamous cell carcinoma, and two animals developed mixed tumors. |
Hindy et al.15 |
|
|
DMBA dissolved in acetone
|
Intraglandular injection after surgical exposure |
1 mg (1 single dose) |
50 weeks |
Not specified |
Sarcomas predominated over carcinomas (>80%) |
Idea et al.21 |
|
|
DMBA dissolved in olive oil |
Intraglandular injection after surgical exposure |
0.02 mg (1 single dose) |
17 weeks |
Decrease/absence of granules in convoluted tubule cells. Ductal structures with cystic cavities. Hyperplasia. Squamous metaplasia of epithelium |
Squamous-cell carcinomas with varying degrees of keratinization; fibrosarcoma and mixed carcinoma. One malignant pleomorphic adenoma and one cystic adenoma |
Takai et al.11 |
|
|
DMBA dissolved in corn oil (95%) |
Subcutaneos injection into the submandibular area |
0.5 mg (1 single dose) |
13 weeks |
Not specified |
Carcinoma, sarcomas, and mixed tumors |
Table 1 Use of DMBA to induce salivary glands carcinogenesis in rats and mice
Regarding the histological findings in the glands, the use of DMBA granules or pellets in both rats and mice, was associated with a higher percentage of animals with tumors (greater than 80%). Taking samples at different times allowed to observe the progression of the lesions. In the early stages (4–6 weeks after DMBA injection), it was associated with squamous metaplasia and keratinization in the area of the implant site with the appearance of preneoplastic lesions; and necrosis was also evident in some metaplastic islands. Regarding the presence of tumors, they were more often after eight weeks. In the four reviewed articles, the presence of carcinomas was mainly reported; adenomas and sarcomas were less frequent (the latter were reported to appear later). The injection of DMBA dissolved in acetone was also associated with a high percentage of animals with tumors (more than 80%). Three articles reported experiments with rats. As it was described before, the article of Zaman, et al.13 reported 100% of female rats with adenocarcinomas, being the 50% of them associated with fibro sarcomas. It was also observed the predominance of fibro sarcomas in male rats, and no metastasis in the lymph nodes and lungs were described. The other two articles showed results obtains with male rats in which, taking samples at different time points, allowed the investigation of carcinogenesis from the beginning, with inflammatory changes, until the tumor presence. As early injures, it was described some cases of chronic Sialadenitis and ductal cell atypia. Most animals developed carcinoma after fifteen weeks, and there were reported less cases of squamous cell carcinoma and sarcoma. The use of mice as an experimental model was associated with predominance of sarcoma over carcinoma.
Development of an experimental model of DMBA-induced carcinogenesis in mouse’s salivary glands
Considering that mice are one of the most used rodents for the development of experimental models, especially due to their size, we developed a model of submandibular salivary gland carcinogenesis by chemical induction with a single dose of DMBA. BALB/c mice were anesthetized, shaved the ventral skin of the neck, and the left submandibular gland was exposed through an incision of 2 cm in the skin. The carcinogen was injected (2%) dissolved in corn oil. Once the surgery was finished and the suture was performed, the mice were maintained in a special room until they woke up from the anesthesia. After that, the animals were controlled weekly for 12 weeks and all observable changes, including changes in behavior, appearance of skin lesions and palpable tumors, were recorded. Observation of the mice showed swelling in the area of surgery during the first 2 weeks. From week 5, the mice presented internal hardness and hair loss in the glandular area. Some animals (50%) presented palpable and measurable tumor masses from week 10. There were no symptoms for which sacrifice was required before the scheduled time; thus, all mice were sacrificed at the end of the experimental period (12 weeks).
Salivary glands’ samples were processed with routine techniques, paraffin embedding, cutting and staining with H&E.
Microscopic observations showed that 89% of the mice developed malignancies. It was observed the presence of in situ carcinoma, microinvasive and invasive carcinomas (75%), sarcomas (12.5%), and carcinosarcomas (12.5%). Some animals presented in situ carcinoma at the ductal level, with ductal ectasia and fibrosis (Figure 2). Figure 3 shows an invasive ductal carcinoma with areas of microinvasion. Other carcinomas presented glandular appearance (Figure 4). Another characteristic was that carcinomas presented squamous areas with cellular pleomorphism (Figure 5); in some of them, the sarcoma component was associated with skeletal muscle infiltration (Figure 6). Associated lesions were also observed in most animals; atypical ductal hyperplasia in all the mice (100%), periductal fibrosis in 87.5% of the samples, and sarcomatous stroma in 62.5 % (Figure 7). Considering that angiogenesis is a characteristic closely related with the growth of different types of tumors, it was evaluated in the samples obtained at the end of the experimental period. A characteristic of the tumors was that 50% of them presented more than 5% of their area occupied by blood vessels. The percentage of blood vessels was 5.4 ± 2.6% and 4.8 ± 2.9% for carcinoma in situ and microinvasive, respectively (Figure 8).

Figure 2 In situ carcinama in mouse submandibular gland injected with DMBA.
A; In situ carcinoma at the ductal level, ductal ectasia and fibrosis in other adjacent ductal structures (100X), B; In situ ductal carcinoma associated with stroma reaction (400X).

Figure 3 Carcinoma in mouse submandibular gland injected with DMBA. A; Presence of acinal atrophy and invasive ductal carcinoma. The skin is not compromised (25X), B; Areas of microinvasion (400X), C; Ductal lumen and invasive ductal carcinoma (400X).
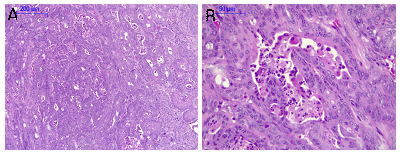
Figure 4 Carcinoma in mouse submandibular gland. A; Carcinoma with a glandular appearance (100X), B; Carcinoma at higher magnification with a glandular appearnce and comedonecrosis (400X).
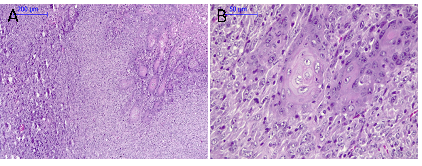
Figure 5 Carcinosarcoma in mouse submandibular gland. A; Squamous areas and spindle cell areas in facicles (100X), B; Squamous areas at higher magnification; cellular pleomorphism is observed (400X).

Figure 6 Carcinosarcoma in mouse submandibular gland. A; Sarcoma component. Cellular pleomosrphism and atypical mitosis (400X), B; Sarcoma component with pleomorphism and skeletal muscle infiltration (400X) is observed (400X).
The descriptive review is a useful tool to evaluate the available evidence; in this article, to analyse the ability of the carcinogen DMBA to induce salivary gland carcinogenesis by using different animal models. Analysis of the results of several individual studies showed that rodents are the most used models; rat and mouse being the ones with the most reported studies. However, although some works date back several years; it is not observed that a particular model has won over others used. The mouse is often chosen due to its size; however, regarding induction methodologies, there is no uniformity of criteria. Intraglandular injection is presented as the most effective method, although the way of administering the carcinogen is not the same (some use granules, others in solution), and the results obtained are not uniform either.
These previous results allowed us to see the potential of the animal model in mice and thus, in this work we proposed to develop a simple model of carcinogenesis induction with DMBA in mice; studying its most outstanding characteristics. Our study demonstrated that the injection of the carcinogen DMBA directly into the submandibular gland, exposed by surgical incision, was an optimal technique to induce the tumor at the desired site. This marks a difference with the subcutaneous injection technique, without surgery, which does not allow the carcinogen to be adequately directed to the gland. Furthermore, it is worth highlighting the use of a single injection of DMBA in solution, which was sufficient for the development of the tumor, making this a relatively simple model with high positivity. The histopathological results showed the existence of associated non-neoplastic lesions and different stages of carcinoma: In situ, microinvasive and invasive, which constitutes an excellent model for the study of salivary gland neoplasms. In addition, the possibility of taking samples at intermediates time would allow the study of tumor progression. One limitation is the lack of myoepithelial cell proliferation demonstrable by the absence of biphasic neoplasms with ductal epithelial and myoepithelial components.
The histopathological findings of associated non-neoplastic lesions, preneoplastic, in situ, incipient and advanced neoplastic stages (atypical hyperplasia, dysplasia, carcinoma in situ, microinvasive carcinoma, and invasive respectively) show that the model allows, with a single injection of the carcinogen DMBA, the study of the progression of salivary gland neoplasms. In addition, the presence of carcinomas of ductal origin and carcinosarcoma is highlighted, both histopathological types of tumors are described in samples from human salivary glands.
This study was financed by Consejo de Investigación de la Universidad Nacional de Tucumán (PIUNT J613/1), and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina (PIP 2022-0280).
The authors thank Nicolás Martínez, a Dentistry student who made the diagrams to illustrate the model.
The authors declare that there are no conflicts of interest.

©2024 Carino, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.