International Journal of
eISSN: 2573-2889


Research Article Volume 4 Issue 4
1Department of Water Pollution Research, National Research Centre, Egypt
2Department of dairy, Food industry and nutrition Division, National Research Centre, Egypt
Correspondence: Mohamed NF Shaheen, Environmental Virology laboratory, Water Pollution Research Department, Environmental Research Division, National Research Center, 12622 Dokki, Cairo, Egypt, Tel +2 01016710071
Received: August 08, 2019 | Published: August 30, 2019
Citation: Shaheen MN, Rizk NM, Allayeh AK, et al. Enterobacter cloacae inhibits human rotavirus infectivity in vitro. Int J Mol Biol Open Access. 2019;4(4):154-156. DOI: 10.15406/ijmboa.2019.04.00112
Rotavirus (RV) is a major agent of acute gastroenteritis in human worldwide. Currently no efficient drug to inhibit RV gastroenteritis and vaccines remains the only available strategy to prevent and control RV infections. This study aimed to investigate the effect of Enterobacter cloacae on RV infections in vitro. For this purpose, the cytotoxic effect of this bacterium on cell culture was tested then the antiviral assay was conducted using different concentrations of E. cloacae and strategies to understand the mechanism by which E. cloacae can inhibit RV infections. Our results demonstrated that E. cloacae was safe to cells at concentration up to 1.00E+06 CFU/ml. The Highest antiviral activity was observed from E. cloacae at concentration 1.00E+06 CFU/ml when the cell lines were treated with the E. cloacae plus RV together at the same time (competition assay) and when the cell lines treated with E. cloacae before RV infection (pre-treatment assay), protecting 62% and 55% of cell line, respectively, with reduction in virus titers by 1.5 log10TCID50/ml and 1.3 log10TCID50/ml, respectively. Our findings revealed the potential of E. cloacae in inhibition of rotavirus infections.
Keywords: Enterobacter cloacae, rotavirus, gastroenteritis, in vitro
Acute diarrhea is a common and important cause of childhood death and mortality in developing countries. 1 Gastrointestinal diseases are one of the leading causes of mortality in children less than 5 years of age, accounting for almost 10% of mortality in this age group.2–5
Rotavirus (RV), within the Reoviridae family, is a non-enveloped virus with 11 segments of double-stranded RNA and it is classified into 50 P types and 35 G on the basis of VP4 (protease-sensitive) and VP7 (glycoprotein) protein,6 respectively. RV represent the most important etiologic agents of viral gastroenteritis in infants and young children, as well as many young animals worldwide.7 It is also the major viral agent of acute gastroenteritis in children<5 years of age, which may lead to death in severe cases.8 The primary rout of RV transmission is the fecal-oral route via person-to-person contact or swallowing of fecally contaminated water and food, with waterborne being one of the most important exposure pathways. 9–11 Currently no efficient drug inhibit RV infections and vaccines remains the only effective and economical means to control and prevent RV infections.12
It has been documented that human norovirus-like particles interacts with E. cloacae SENG-6 isolated from a stool specimen of a healthy person through extra cellular polymeric substances (EPS) where histo-blood group antigen (HBGA)-like substances were localized.13 In addition, Amarasiri et al., 14 reported that HBGA-like substances excreted in the EPS of E. cloacae SENG-6 displayed the strain dependent recognition and removal of human norovirus-like particles. Therefore, HBGA positive bacteria-virus system can provide an excellent platform to investigate the contribution of specific interactions on human enteric virus survival and removal. In this study, we studied the effect of E. cloacae on rotavirus infectivity in vitro.
Preparation of bacteria
cloacae was activated by three successive transfers in modified MRS followed by three successive transfers in sterile 10% reconstituted skim milk powder and incubated at 37°C. Then plated on MRS media and incubated for 24 h at 37°C, selected one separate colony in tryptone soy broth to start serial dilution to prepare different bacterial concentrations.Cell lines and virus
MA 104 cell line (African green monkey kidney) was purchased from VACSERA (Holding Company For Biological Products And Vaccines, Egypt) and cultivated in cell culture flasks (75cm2) in minimal essential medium containing Eagle's salts (MEM) supplemented with 5% fetal bovine serum, 1% antibiotics and antifungal (PSA-penicillin G Cultilab 100U/mL/100 g streptomycin sulfate/mL /Amphotericin B 0.25g/ mL). All chemicals required for cell line growth were purchased from Lonza, Belgium. Simian rotavirus SA-11 stock was pre-activated with trypsin 10 mg/ml trypsin for 30 min at 37°C. The diluted tenfold of activated RV stock was replicated in MA 104 cells and the cytopathic effect was checked after 72h of incubation. The 50% tissue culture infectious doses/0.1ml (TCID50/0.1ml) was estimated as described previously by karber method (karber, 1931), then stored in small aliquots at – 80°C until used.
Cytotoxicity assay
The cytotoxicity of E. cloacae on MA 104 cell lines in 96 well plate was conducted using a MTT [3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide] assay as described previously by Lee at al.15 In summary, MA 104 cell lines were cultivated in 96-well microplates with suspensions of 100μl containing various amounts of bacterium ranging from 1.00E+03 to 5.00E+07 CFU/ml then incubated for 48 h. Untreated control cells were also included. After that, the medium was discarded and replaced by 100μL of fresh culture medium containing MTT reagent in each well. After incubation period at 37°C for 4 h, culture medium containing MTT reagent was discarded and 100μL of dimethylsulfox-ide (DMSO) was added to each well. Cell Viability were then measured using a Spectrophotometer reader (Asys Expert Plus microplate reader, UK) at 570nm. The survival rates of cell survival rate was calculated as the average OD value of bacteria treated cells/ average OD value of cell control. The 50% cytotoxic concentration (CC50) isdefined as the bacterial concentration that can decrease 50% of cell viability when compared with cell control. Three non-cytotoxic concentrations of bacterium were used for next bacterium-virus interaction assays.
Effect of probiotic bacteria on RV infection by MTT assay
MA 104 cell lines were cultivated in 96-well plate. After incubation at 37°C for 24h under CO2 atmosphere, the culture medium was removed and the bacterium-virus interaction was carried out in four different experimental protocols. Three experimental protocols were conducted to study the effect of bacteria on the host cells while the fourth protocol (virucidal) is to investigate the direct effect of bacteria on the viral particles as mentioned in our previous work.16
Pretreatment
The cell medium of 96-well plates was discarded and after washing of the cell monolayers twice with phosphate buffer saline (PBS), 100μl cell medium containing bacteria was transferred onto the cell monolayers for 1.5 h at 37°C under 5% CO2 condition. After that, the non-bounded bacteria were rinsed with PBS and challenged with 100μl of pre-activated RV at 106 TCID50/ml for 1 hr at 37°C under CO2 atmosphere. After discarding the viral suspension from wells, the cell monolayers was rinsed twice with PBS and incubated with FBS free DMEM with trypsin (1%).
Competition assay
The pre-activated RV was transferred together with E. cloacae onto the cell monolayers for 1 h at 37°C in a 5% CO2 atmosphere. After removal the mixed solution, the cell monolayers were rinsed twice with PBS then incubated with FBS free DMEM with trypsin (1%).
Post-treatment assay
cloacae was inoculated onto the cell monolayers for 1.5 h at 37°C under 5% CO2 atmosphere. After removal the unbounded bacteria, the cell monolayers were rinsed twice and the pre-activated RV was transferred onto the cell monolayers for 1 h at 37°C in a 5% CO2 atmosphere. Then the culture medium containing unabsorbed virus was discarded and replaced with FBS free DMEM with trypsin (1%).Virucidal assay
100μl of E. cloacae was incubated for 1 h at 37°C with same volume of pre-activated RV. After that, the cell monolayers were inoculated and incubated with the virus-bacteria mixture. In the four experimental approaches, the virus control (cell culture plus virus suspension) and cell line control (cell culture plus medium) were included. To investigate the inhibitory effects of E. cloacae on viral replication, the percentage of cell viability was determined spectrophotometrically by MTT assay as described by Ren et al., (2011) as follows: Percent viable cells = as [(ODa-ODv)/(ODm-ODv) X100, where ODa is the OD value of bacterial group; ODv is OD value of virus control, and ODm is the OD value of blank control. The results were confirmed by TCID50 metod as described by Reed and Muench (1938). In brief, one non-toxic concentration of E. cloacae with 10-fold dilution of RV were used to inoculate the cell monolayers in four different ways as described above with MTT method. Quadruplicate wells were used for each concentration of the virus. The difference between the values of virus with bacteria and in absence of bacteria is equal to virus titer reductions.
Statistical analysis
To evaluate the possible correlation between viral and bacterial in samples, a Pearson correlation and linear regression test, a two way ANOVA test and a Student’s t test were performed using Graph Pad Prism 5.0 (USA); data were considered statistically significant at a P-value ≤ 0.05.
To determine the effect of E. cloacae on RV infection, MA 104 cell lines were infected with virus before, during, after, and at same time of cell treatment with three non-toxic concentrations of the bacterium to understand the mechanism of bacterium-virus interactions. Before using the bacterium in the antiviral studies, the non-toxic concentrations was defined where E. cloacae was safe to cells at concentration up to 1.00E+06 CFU/ml. At this concentration, the viability rate of MA 104 cell lines was 100% where no morphological changes were observed on the bacteria-treated cells, when compared with cell control. As shown in Figure 1, the cell viability of the RV infected MA-104 was great at the highest concentration of E. cloacae (1.00E+06 CFU/ml), protecting 55%, 62%, 47%, and 54% of RV infected MA-104 during pre-treatment, competition, post-infection, and virucidal assays, respectively.
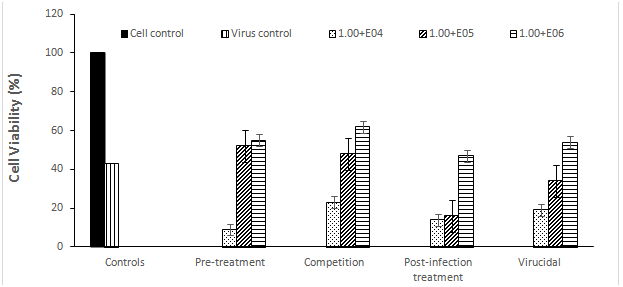
Figure 1 The percentage of cell viability under rotavirus infections with different concentrations of E. cloacae in MA-104 cell lines by MTT assay.
The protection rates decreased to 52%, 48%, 16%, and 34% with decreasing the bacterial concentration to 1.00E+05 CFU/ml during pre-treatment, competition, post-infection, and virucidal assays, respectively. Lowest protection rates were occurred with decreasing the bacterial concentration to 1.00E+04 CFU/ml, recording 9%, 23%, 14%, and 19% during pre-treatment, competition, post-infection, and virucidal assays, respectively. In agreement with MTT results, TCID50 results were obtained. At the higher concentration of bacteria (1.00E+06), a reduction in virus titers reached to 1.3, 1.5, 0.8, and 1.1log10TCID50/ml during pre-treatment, competition, post-infection, and virucidal assays, respectively (Figure 2).
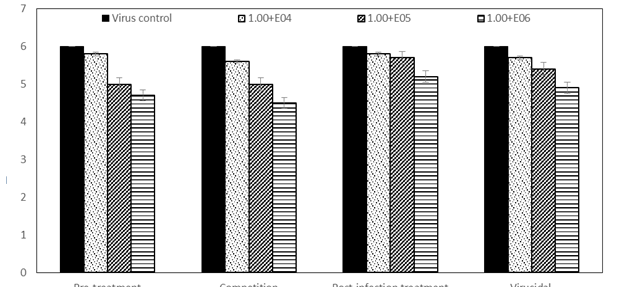
Figure 2 The percentage of cell viability under rotavirus infections with different concentrations of E. cloacae in MA-104 cell lines by TCID50 assay.
The reduction rates decreased to 1, 1, 0.3, and 0.6 log10TCID50/ml during pre-treatment, post-infection, and virucidal assays, respectively, with decreasing the bacterial concentration (1.00E+05 CFU/ml). The reduction in virus titers at the lowest concentration of bacteria (1.00E+04 CFU/ml) was not significant as 0.2, 0.4, 0.2, and 0.3 log10TCID50/ml during pre-treatment, competition, post-infection, and virucidal assays, respectively. To our knowledge, this is the first in vitro study to evaluate the effects of E. cloacae on rotavirus infectivity. However E. cloacae has been reported to inhibit other enteric viruses such as norovirus infectivity in neonatal Gn pigs.17 There are some reports that HBGA bacteria interact with norovirus and rotavirus, enhancing their replications.18 However, a clear differences was found between rotavirus strains in their ability to use HBGA as receptors show that HBGA may not be essential in host cell invasion.19
In conclusion, the E. cloacae was able to induce protective effects against acute rotaviral infections in vitro, which might through direct interaction with viral capsid, blocking the virus receptors on the cell surface, and/or interference with the virus life cycle inside the host cells. Further studies in vivo would be conducted to confirm these results.
None.
The author declares there is no conflicts of interest.

©2019 Shaheen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.