International Journal of
eISSN: 2573-2889


Research Article Volume 7 Issue 1
Medical Pathologist-Oncologist, Department of Pathology, Nicaragua
Correspondence: Venus Maria Tapia Lopez, Medical Pathologist-Oncologist. Department of Pathology, Hospital Escuela Bertha Calderón Roque, Managua, Nicaragua, Tel +505 8615 7301
Received: March 03, 2024 | Published: March 12, 2024
Citation: Lopez VMT. Early-onset breast cancer: a look from molecular biology. Int J Mol Biol Open Access. 2024;7(1):35-39. DOI: 10.15406/ijmboa.2024.07.00164
Breast cancer is the most frequent invasive cancer in women, affecting one out of every 8 women during their lifetime, and it is the leading cause of cancer-related death in women worldwide. Young women represent only 3% of all diagnosed cases; however, these patients have larger tumors and a higher proliferation rate compared to older women, and early-onset breast cancer also expresses more aggressive histological and molecular subtypes with a worse prognosis. In general, breast cancer in young women is related to a more aggressive biology and a worse prognosis. Tumors are mostly aggressive and have a worse clinical course. Basal type tumors and HER2-positive cancers are most often diagnosed in young women with breast cancer. Breast cancer was 34.3% and 22%, respectively, with a higher frequency of lymph node metastasis, multifocal disease and high tumor grade, compared to the older population. They are generally diagnosed at an advanced stage. All these characteristics act as poor prognostic factors increasing the mortality rate up to 1.5 times and higher recurrence rate compared to older patients. In studies analyzing the gene expression of tumors in the group of young patients, up to 63 genes have been identified that are specifically altered, through which the molecular pathways that are affected can be studied, being specific oncogenic alterations different from those that promote tumorogenesis in older patients.
Keywords: early onset cancer, genetic mutations, molecular pathways
Each year 1.67 million women are diagnosed with breast cancer and 522,000 die from this disease worldwide. In developing countries, 5-year survival is 30% to 45%, compared to 80% survival in developed countries, which is due to the time of detection of the disease. In developing countries, more than 53% of cases are detected in advanced stages and the time interval is vital for the prognosis; an interval of up to 3 months has been found from the patient's first contact with medical attention to the definitive diagnosis and correct management of her disease.1 Breast cancer is a disease characterized by diverse clinical behaviors and different biological characteristics, making the process of diagnosis, prediction, prognosis and management more challenging for physicians, surgeons, pathologists and oncologists, because it presents different molecular profiles.
The cancer generally affects women over 40 years of age. However, in younger women (<40 years) it accounts for 6%- 10% of all breast cancer cases in developed countries and 20% in developing countries. Mortality rates represent 7% vs. 14% for developed and developing countries, respectively. Age is considered, per se, a risk factor associated with breast cancer, in addition to representing a prognostic factor, with a worse prognosis in younger women. The most frequently presented molecular profile by immunohistochemistry in women under 40 years of age is the triple negative subtype (estrogen and progesterone hormone receptor negative and HER2 NEU negative), whereas in postmenopausal patients (60-69%) the most frequently reported incidence is the Luminal A subtype (estrogen and progesterone hormone receptor positive and HER2 NEU negative), which represents a better prognosis for these patients. In addition, high-grade histologic subtypes with a higher proliferation index are more frequently described in younger women.2
Mammography has proven to be a very effective tool for breast cancer screening in older women, but it is not an adequate tool for cancer detection in younger women, because they have dense breast tissue and it limits the detection of tumors or calcifications. Due to the lack of adequate screening tools, younger women are often diagnosed at a late stage of the disease compared to older women. There is much less information in the scientific literature on the prevalence of breast cancer immune phenotypes in young patients than on breast cancer immune phenotypes in all age groups. Traditionally, this type of neoplasm at young ages has been associated with high recurrence rates and lower survival, so it would be expected to find tumors with aggressive phenotypes. Topics addressed include: Etiology; molecular profiling, histopathologic diagnosis, genes involved, and molecular pathology of early-onset breast cancer.
Development
Mammary gland, histological structure
The breast has two types of epithelial cells, two types of stroma and two main structures.
Epithelial cell types: The two types of epithelial cells present in mammary tissue are luminal cells and myoepithelial cells (Table 1). In addition, precursor progenitor cells or stem cells may be present, the latter requiring the application of special techniques for their recognition; they may be referred to as intermediate or basal cells, which can give rise to luminal and myoepithelial cells, thus supporting the theory of clonal neoplasms composed of both cell types (e.g. myoepitheliomas, adenoid cystic carcinoma). They are generally immunoreactive for high molecular weight cytokeratins (cytokeratins 5/6), seen in epithelial hyperplasia, which proves the presence of a mixed population of multiple cell types.3 Luminal cells: Form the innermost layer lining ducts and acini. Luminal cells in the terminal lo-bulillar duct unit produce milk. Luminal cells in the larger ducts do not undergo lactation. Cells are cuboidal to columnar in shape, nuclei are small, round to oval, usually have discrete nucleoli. The cells have a moderate amount of eosinophilic cytoplasm. These cells express low molecular weight luminal keratins.7,8,18,19 They may also express basal keratins.3 Luminal cells are precur- sorous cells of most breast carcinomas.4
|
Cell type |
Function |
Protein expressing |
Injury |
|
Luminal cells |
Terminal lobular duct unit: Milk production. Ductal: milk ducts |
Luminal keratins 7, 8, 18, E cadherin, estrogen receptor and progesterone receptor. |
Usual epithelial hyperplasia and atypical ductal hyperplasia, most carcinoma. |
|
Myoepithelial cells |
Present in the basement membrane, it maintains the polarity of the luminal cell, contraction for milk ejection. |
Basal keratins 5/6, 14, 17, P-cadherin,muscle markers, p63, CD10, podoplanin (D2-40) |
Myoepitheliomas, collagenous spherulosis possible subset of triple-negative carcinomas |
|
Stromal fibroblasts and myofibroblasts |
Support epithelial cells and provide most of the mammary volume. |
CD34 (majority), markers muscle fibroblasts (myofibroblasts), estrogen and progesterone receptors (myofibroblasts), estrogen and progesterone receptors (myofibroblasts) |
Pseudoangiomatous stromal hyperplasia (PASH), fibromatosis, myofibroblastoma Fibroadenoma/ Phyllodes tumors |
Table 1 Cell types in breast tissue and protein expression. Twelves D et al3
Clinicopathologic features of breast cancer in young women
The European Society of Breast Cancer Specialists (EUSO- MA) defines breast cancer in young women as a group of women with breast cancer under 40 years of age. Women under 40 years of age have their own characteristics such as the desire for fertility, pregnancy and breastfeeding, unlike women over 40 years of age, which implies a different approach and establish differences between older, peri and pre-menopausal women.5 Breast cancer in very young women is characterized by being typically more aggressive, the tumors are macroscopically larger, Figure 1 more undifferentiated, with greater lymph node involvement, overexpression of HER2 and absence of hormone receptors, all of which confers a poor prognosis. The 5-year survival rate is 83% for younger women, lower than the survival rate for post-menopausal women. The St. Louis Conference Gallen 1998, established that, ages below 35 years act as a variable with prognostic value.5 Breast cancer in younger women has a higher proportion of history of breast cancer in family members compared to breast cancer in older patients (24% vs. 17%). Breast cancer is a heterogeneous disease. Its prognosis is associated with different classic morphologic factors such as tumor size, histologic grade, axillary node status, vascular invasion, presence of neoplasia and distance to resection margins. Some of these factors are included in the TNM staging system, which allows patients to be grouped according to the level of tumor progression, which is related to the probability of recurrence and, therefore, to the prognosis. It also contributes to decision-making regarding the most appropriate treatment.

Figure 1 Macroscopic image: Mastectomy for right breast cancer, in a 33-year-old female patient. Gross description: Tumor of 10x8x6cm, multifocal, located in outer quadrants, protruding and ulcerating skin, with extensive necrosis.
Source: Hospital Escuela Bertha Cal- derón Roque clinical file.
From a histological point of view, breast infiltrative neoplasms have been classified according to well-established criteria by the World Health Organization (WHO) into different groups: epithelial, mesenchymal, mixed, lymphoid or metastatic origin.6 There is a wide spectrum of morphologic phenotypes and specific subtypes, each with its own particular clinical and prognostic presentation. Non-specific ductal in-filtrative carcinoma (NOS) is the most frequent histologic type, accounting for 75% of all tumor types.
Molecular studies in young women with breast cancer:
Over the decades the mechanisms involved in breast cancer have been extensively investigated, however, there are still challenges in establishing an early and specific diagnosis of each patient, to determine and predict their response and acquired resistance to treatment. Lin et al,7 found that in women younger than 35 years, TP53 mutations, Ki67 percentage and HER2 expression have greater prognostic value compared to the expression of hormonal markers, which are generally negative in this age group. The biomarkers used for breast cancer in general may not be entirely suitable for very young women with breast cancer.7 Anderson and Matsuno, based on the distribution of the age of diagnosis and its mortality rate, defined breast cancer as a mixture of at least two types of cancer. The first would have its age of onset around 50 years of age and the second would occur in women over 65 years of age and could be defined as pre- and post-menopausal.8 There is different gene expression in breast tumor tissue according to age, separating the profiles into very young women (younger than 35 years), young women (35 to 45 years), pre-menopausal women (45 to 55 years) and older women (55 years and older). Analyzing separately the distinctive profiles of each group, they found that the enriched biological processes associated with deregulated genes in very young women (under 35 years of age) included cell cycle control, morphogenesis, proliferation and cell death, among others. Similar to the profile obtained for young women (aged 35 to 45 years), biological network analyses indicated alterations in MAPK, PI3K/Akt and NFkB signaling pathways, in addition to revealing IL1RN, ESR1 and the ERBB2 family genes9 as potentially important.
Germline mutations
Acquired germline mutations in DNA repair and tumor suppressor genes are the most common form of genetic susceptibility to breast cancer, leading to the accumulation of mutations in cell cycle checkpoint and oncogenes that are required for aberrant cell division. About 10% to 20% of early breast cancer cases are hereditary, BRCA 1 and BRCA2 are the most common mutated genes linked to breast cancer since their discovery in the early 1990s.10 Mutations associated with the development of cancers are often classified as high, intermediate and low penetrance mutation based on their relative risk for the specific cancer. BRCA1, BRCA2, TP53, PTEN, STK11 and CDH1 are considered high penetrance mutations and account for 20% of the inherited risk.
Followed by mutations of moderate penetrance, including PALB2, BRIP1, ATM, CHEK2, and RAD51C, which account for about 5% of the inherited risk. In addition, more than 180 mutations are considered low risk for breast cancer, accounting for only 18% of the familial risk. All of these relative risk ratios define only half of the genetic risk for breast cancer, with the other half still unknown.10 BRCA1, a tumor suppressor gene, encodes a 220 KDa protein in response to double-strand breaks (DSBs) by homologous recombination (HR). The BRCA1 mutation causes defect in homologous recombination-mediated repair and loss of function to inhibit tumor tumorigenesis. Although BRCA1 mutation is rare in sporadic breast cancer, but reduced nuclear expression of BRCA1 in breast cancer tissue is common.11
Xiang et al,12 found that 69% of cases were negative for BRCA1 IHC staining in 101 breast cancer patients, and also had increased Ki-67 expression. Reduced BRCA1 expression was also associated with advanced disease stage and lymph node metastasis, high-grade histology, larger tumor size, vascular invasion, negative estrogen and/or progesterone receptor, androgen receptor expression, and positive p53 expression. Bogdani et al,13 found that the absence of BRCA1 nuclear staining was more frequent in younger women than in older patients. Absent or weak nuclear BRCA1 expression appears to be a predictor of poor prognosis.11 (Figure 2)
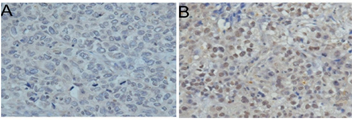
Figure 2 Immunohistochemical expression of BRCA 1 in young women with breast cancer. (A) Negative nuclear expression, (B) Positive nuclear expression Jin-Tao Wei1 et al11
Somatic mutations
In different studies, by next generation sequencing (NGS), the most frequently found pathogenic mutations in young breast cancer patients were PIK3CA and TP53, followed by BRCA2 and PTEN mutation in 5% of frequency. Other mutations detected in lower percentages in these patients by NGS include: ATM 2%, AKT 4%, CHEK2 4%, NRAS 2%, CDKN2A 2%, NF1 2%, RB1 2%, FGFR1 2% and ERBB2 2%.14
TP53
In young female breast cancer, there is a high mutational burden of TP53 that affects the rate of GAS7b transcription; considering the role of GAS7b in regulating cell structure and migration, this aberrant transcription may contribute to metastatic events. This remains to be further investigated.10 Germline TP53 variants are the genetic cause of Li-Fraumeni (LFS), a hereditary cancer predisposition syndrome associated with very early-onset female breast cancer, commonly before the age of 31 years. The pathogenic mutation c.824G>A located in coding exon 7 of the TP53 gene has been identified as a mutated gene in the pathogenic germline TP53. Young women carrying these variants have a high risk of developing breast cancer after the second decade, which rises to 20%-30% below the age of 31 years, peaks between 25 and 35 years and drops after 40 years of age. Young women who carry germline mutations in the TP53 gene have a cumulative risk of 5-8% of developing breast cancer before the age of 30. Germline mutations of TP53 have been shown to be associated with tumors characterized by high grade, HER2 overexpression in 60-83% of cases and multifocality, which histopathologically characterizes breast cancer in young women. In general, TP53 germline testing should be routinely applied to all patients diagnosed with invasive breast cancer or ductal carcinoma in situ (DCIS) before the age of 31 years.11
PI3K/Akt/mTOR signaling pathway
This intracellular pathway has attracted much interest in recent years as a potential therapeutic target and to determine its role in the different subtypes of breast cancer among younger patients. Different studies in patients with early- onset breast cancer have shown that there is altered immunohistochemical expression of any of the components of the IGF1R, PTEN/Akt/mTOR pathway among young patients. The overexpression of IGF1R in the Luminal B- like/HER2+ subtype and the loss of PTEN expression in the TN/Basal subtype supports that alteration of this pathway is related to the pathogenesis of these molecular subtypes, and in turn, its potential relevance as a therapeutic target should be considered in the near future.6 This intracellular molecular pathway plays a key role in the regulation of cell survival, proliferation, migration, apoptosis, metabolism and angiogenesis. Phosphatidylinositol-3-kinase (PI3K) is a family of enzymes whose functions are to phosphorylate the hydroxyl group at the 3' position of the inositol ring in the plasma membrane and generate important second messengers. We currently know of three different classes of PI3Ks, which in turn are divided into subclasses according to their affinity for certain substrates, their homology and the functions they perform. Class I is the best characterized in terms of its involvement in cancer pathogenesis and its PI3Ks are divided into subclass IA and IB. PI3K class IA consists of one of three catalytic isoforms (p110a, b, and d) encoded by the PIK3CA, PIK3CB, and PIK3CD genes, and a regulatory subunit, which can be p85a or its splicing variants (p55a and p50a), p85β or p55g. In addition, the catalytic subunit, p110a, can be regulated by TK receptors, growth factor binding receptors and also by G protein-associated receptors. PI3KCA activating mutations in various hot spots occur most frequently at positions E542K, E545K and H1047R. These constitute more than 80% of the total number of mutations described in PI3KCA in breast cancer. The results of in vitro and X-ray crystallography studies suggest that mutations in E542K and E545K (exon 9) abrogate molecular interactions, whereas mutations in exon 20 (H1047R domain) produce constitutive activation of the gene. In studies with transgenic mice, genetic or pharmacological inactivation of PIK3CA (H1047R) expression results in the disappearance of mammary tumors. However, some of them recur and can become resistant to PI3K inhibition via the c-myc pathway. Other studies have shown that mutations in exon 20 of PIK3CA are relatively frequent, occurring in up to 23-33% of HER2 subtype tumors.6
In addition to its activity to promote cell growth and survival, the PI3K pathway interacts with estrogen receptors (ER) directly and indirectly since it can be activated by ER. In addition, activation of the PI3K pathway can lead to anti- estrogen resistance, as has been demonstrated in various experimental models, which can be reversed after inhibition of the PI3K pathway. Therefore, some studies suggest that the combination of antiestrogens and PI3K pathway inhibitors may be a more clinically effective strategy than single antiestrogen treatment.6 It has been shown that activating mutations of PIK-3CA cause resistance to anti-HER2 treatments such as Trastuzumab®, due to additional activation in this pathway, independently of HER2/HER3 dimers. It has also been described that HER2-positive tumors are sensitive to PI3K and mTOR inhibitors, both before and after acquiring resistance to treatments with Trastuzumab® and/or Lapatinib® (dual HER1/HER2 inhibitor).6 Activation of mTOR in breast cancer has been described to be associated with tumors with an aggressive phenotype. To date, there have been a few studies that have analyzed the status of mTOR and mTOR activation in breast cancer.
The effect of these treatments in postmenopausal women with breast cancer. However, there are few papers in the literature analyzing the status of mTOR in the subgroup of young women. Recently, it has been suggested that mTOR is a potent effector in Lapatinib treatment. The relationship between resistance to antiestrogen treatment in ER-positive breast tumors and mTOR activation has also been established. Inhibition of mTORC1 causes negative feedback on all receptors that can activate the PI3K pathway (IGF1R, IRS-1 and HER3) suggesting that direct inhibition of the pathway can be very effective.15
miRNAs in breast cancer
The role of miRNAs in the onset of breast cancer in young women is poorly understood. Two similar studies relate the presence of a functional polymorphism in the miR-146a gene with an advance in the age of onset of breast cancer. Similarly, a polymorphism in the miR-502 binding site, located in the 3'UTR region of the SET8 gene, is associated with an earlier age of onset of breast cancer16. A study in Lebanese women with breast cancer revealed an overexpression of miR-155 in patients younger than 40 years old, when compared to those older than 40 years old.17 There is also a reported association between miRNA profile and familial breast cancer not due to BRCA1/2, a type of cancer that originates mainly in young women.18 miRNAs have shown potential prognostic value as well as therapeutic targets. For this reason, since 2005, when deregulation of miR- NAs in breast cancer was first reported, many studies have focused their efforts on investigating the expression of miRNAs in breast cancer and their role in the development of the disease. Gasparini and coworkers,19 state that the combined detection of miRNAs miR-155, miR- 493, miR-30e and miR-27a, together with rudimentary immunohistochemical detection techniques, separates triple-negative breast cancer patients into a high-risk group and a low-risk group.20 The epithelial-mesenchymal transition process is essential during mammalian embryonic development; however, this process has been linked to cancer initiation and progression. It is characterized by the loss of the epithelial marker E- cadherin, cell adhesion proteins and cell polarity. Furthermore, the transition from epithelium to mesenchyme is also important in breast cancer to establish the onset of carcinogenesis, as cancer cells reproduce the behavior of normal stem cells. Yu and coworkers were the first to study miRNA expression in breast cancer stem cells (BCSCs) by comparing miRNA expression in self-renewing BCSCs and differentiated cells from both cell lines and primary breast tumors.19 Of the selected target genes, GRIN2B was validated, whose mRNA expression is found to be overexpressed in young women, in agreement with the expression of miRNAs. The presence of HER4 protein in tumor tissue was significantly higher in young women. Moreover, the staining in these was mainly membrane staining, as opposed to the nuclear staining found in older women.5
Tumor microenvironment
The tumor microenvironment plays an important role in breast cancer tumorigenesis and progression. Targeting malignant and non-malignant components of the tumor microenvironment can help in cancer treatment. Endocrine changes during reproductive age and gestation play a key role in breast alteration. Eight stromal genes were differentially expressed in breast tumors from very young patients (35 years or younger) compared to tumors from older patients (50-65 years) (UQCRQ, ALDH1A3, EGLN1 and IGF1 overexpressed in breast tumors from very young patients (35 years or younger) compared to tumors from older patients (50-65 years) (UQCRQ, ALDH1A3, EGLN1 and IGF1 overexpressed in breast tumors from very young patients (50-65 years). Expressed, while FUT9, IDI2, PDHX and CCL18 are so- bre- expressed.
Early-onset breast cancer has a diverse etiology, involving different pathways of tumorogenesis and genetic mutations. It is mainly related to those pathways that give adverse prognoses, in clinical and histological presentation and molecular classification of high-grade and highly proliferative tumors. Studies are still underway to discover new molecular markers exclusively associated with early-onset cancer, which may help to personalize therapy for this group of patients. In addition, specific genes related to the tumor microenvironment and extracellular matrix proteins are being investigated. Different pathways, especially TP53 mutations may be a promising area for future research, of breast cancer in young women.
To the doctors of the Bertha Calderón Roque Teaching Hospital: Alejandra Yahoska Jirón Ayerdis, Neonatology Service; Alison María García Rivera, Radiology Department; Maryina Feliza Malespín Matamoros, Emergency Service; Karla Valle Carcache, Emergency Service; María Amparo Morales Acuña, Maternal-Fetal Medicine Service; Rita Isabel López Solano, Obstetric High Risk Service; Juan José Almendarez Martínez, Urogynecology Service; Goizeder López Rubio, General Surgery Service.
The author declare that there are no conflicts of interest.

©2024 Lopez. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.