International Journal of
eISSN: 2573-2889


Research Article Volume 5 Issue 3
1Department of Biochemistry, Ahmadu Bello University, Nigeria
2Department of Biochemistry, Federal University Lokoja, Nigeria
3Department of Biochemistry, University of Ibadan, Nigeria
Correspondence: Sheneni Victor Duniya, Department of Biochemistry, Federal University Lokoja, Kogi, Nigeria, Tel 234- 80-3351-9009
Received: April 26, 2020 | Published: September 30, 2020
Citation: Joseph BC, Duniya SV, Sokoato MI. Characterization of essential oils from hyptis suaveolens leaves by gas chromatography-mass spectroscopy and fourier transform infra-red spectroscopy. Int J Mol Biol Open Access. 2020;5(3):125-133. DOI: 10.15406/ijmboa.2020.05.00139
Essential oils were extracted from the leaves of Hyptissuaveolens obtained from Samaru area of Zaria-Kaduna, Nigeria by hydrodistillation. Gas Chromatography-Mass Spectrometry (GC-MS) and Fourier Transform Infra-Red (FTIR)was carried carried-out on the essential oils and the physicochemical properties were determined using standard methods. Percentage yield of the essential oils was gotten to be 0.05.TheGC-MS analysis showed that terpenes are the major organic compound present in the essential oil which was confirmed by FTIR with the O=C-O-Cstretch functional group indicating the presence of terpenes. FTIR analysis shoewed the presences of functional groups such as esters, ethers, carboxylic acids etc which are useful in the making of soap, perfume, anaesthesia and several other things. The physicochemical analysis showedthat the essential oil has the following physicochemical properties; Iodine value 23.59+0.12g/100g, saponification 100.18+0.8mgKOH/g, Peroxide value40.00+0.02meq/kg, Acid value 3.37+0.01mgKOH/g, Ester value 34.30+1.00mg/g and free fatty acids 0.15+0.57%.
Keywords: hyptis suaveolens, essential oils, GC-MS, FTIR
Essential oils are volatile natural complex secondary metabolites characterized by a strong odour and have agenerally lower density than that of water.1 They are natural volatile mixtures of hydrocarbons with a diversity of functional groups, and their repellent activity has been linked to the presence of monoterpenes and sesquiterpenes.2–4 Essential oils are plant products obtained by hydro-distillation or other methods. This complex of compounds are produced by plants, giving them their characteristic smell and taste, and are usually composed of 20–80 or more substances. Their main components are monoterpenes (C10) and sesquiterpenes (C15) derived from isoprene. Although there are exceptions, most monoterpenes present in essential oils have very low toxicity to mammals and are rapidly degraded in the environment. These characteristics and the discovery of the insecticide and repellent effects of some monoterpenes have awoken great interest in the possibility of using these compounds as alternative tools for insect control and generally pest control. Several monoterpenes have been reported as insect repellents. There are 17,500 aromaticplant species among higher plants and approximately 3,000 essential oils are known out of which300 are commercially important for pharmaceuticals, cosmetics and perfume industries. Apart from insecticidal potential they are lipophilic in nature and interfere with basicmetabolic, biochemical, physiological and behavioural functions of insects. Essential oil compounds and their derivatives are considered to be an alternative means of controlling many harmful insects and their rapid degradation in the environment have increased specificity that favours beneficial insects.1 The activity of aromatic repellent plants is due to essential oils present in the plant material. They are used as flavour in food products, odorants in flagrances, pharmaceuticals (antimicrobial,) and as insecticides.2–4 Essential oils of various plants have been identified as potential sources of insecticides which can be evolved into commercial formulations.5,6 A large number of essential oils are known to behave as insecticides/ovicides, attractants/repellents, anti-feedants, juvenile hormone analogues, etc. This has necessitated the need for research and development of environmentally safe, biodegradable, low cost, indigenous methods for vector control, which can be used with minimum care by individual and communities in specific situations.7 Overall, among natural products essential oils are known to exhibit low toxicity to mammals, and the most terpenoids and phenols found in plant essential oils have been approved as flavouring compound in food.8 Essential oils have received much attention as potentially useful bioactive compounds against insects showing a broad spectrum of activity against insects, low mammalian toxicity and degrading rapidly in the environment.9
Hyptissuaveolens (L.Poit) is one of the important traditional medicinal plants belonging to family lamiaceae.10 It is commonly called Bush mint, Bush tea, Pignut, or Chan. It is known generally as Vilayati Tulsi in Hindi, KondaThulasi in Telugu, Bhustrena in Sanskrit, Daddoya-ta-daji (family name) and specifically Sarakuwansauro in Hausa, Efiri (family name) in Yoruba, Nchuanwu (family name) in Ibo, and Tanmotswangi-eba in Nupe.10 The leaves of Hyptissuaveolens have been traditionally used as a stimulant, antisplasmoidic, against colds and diarrhoea. It has been and still in use traditionally to repel mosquitoes by burning the leaves.11,12 Mass spectrometry, coupled with chromatographic separations such as Gas chromatography (GC/MS) is normally used for direct analysis of components existing in traditional medicines and medicinal plants.13 In the last few years gas- chromatography mass-spectrometry has become firmly established as a key technological platform for metabolite profiling in plant, which has proven to be a valuable method for the analysis of non-polar components and volatile essential oil, fatty acids and lipids.14 GC-MS based metabolome analysis has profound applications in discovering the mode of action of drugs or herbicides.14 It has been observed that the chemical composition of EO extracted from Hyptissuaveolens fresh leaves showed several differences with respect to previous studies. It is well acknowledged that the Hyptissuaveolens EO chemical composition and biological activity change as a function of the origin and collecting period of the plants. This is a common feature among secondary metabolites and from essential oils of Lamiaceae plants in particular. Several authors reported a large variability in the composition of this family due to genetic, geographical and seasonal factors.8
Fourier Transform Infra-Red Spectroscopy measuring principle is a measurement with infrared (IR) light. The scan area of the IR wave length by use of the FTIR measuring principle is large. The principle of FTIR is that the gas to be analysed is led through a cuvette with an IR light source at one end that is sending out scattered IR light, and a modulator that cuts the red IR light into different wave lengths. At the other end of the cuvette is a detector measuring the amount of IR light passing through the cuvette. It is the absorption of light at different wave lengths that is an expression of the concentration of gases to be analysed. The total internal energy of a molecule in a first approximation can be resolved into the sum of rotational, vibrational and electronic energy levels. Infrared (IR) spectroscopy is the study of interactions between matter and electromagnetic (EM) fields in the IR region, in this spectral region, the EM waves mainly couple with the molecular vibrations. In other words, a molecule can be excited to a higher vibrational state by absorbing IR radiation. The probability of a particular IR frequency being absorbed depends on the actual interaction between this frequency and the molecule. In general, a frequency will be strongly absorbed if its photon energy coincides with the vibrational energy levels of the molecule. IR spectroscopy is therefore a very powerful technique which provides fingerprint information on the chemical composition of the sample.
Plant material
Fresh Hyptissuaveolens leaves were used in the experiment for the extraction of essential oil via hydro-distillation.
Chemicals and reagents
All chemicals and reagents were of analytical grade.
Equipments
Round bottom flask, water condenser, Erlenmeyer flasks, separating funnel, glass bottles with tight cover, paper tape, Cardboard papers, Aluminium cage with wire gauge, Cage made of Mosquito net with wooden support, plastic containers and petri dishes.
Methods
Plant Material Collection and Identification
The plants were collected from Samaru area of Zaria, Kaduna State. They were then taken to the herbarium, department of Biological Science for identification. Voucher specimen number 2020 wasdeposited there. More of the plant leaves were then plucked and taken to the laboratory for further use.
Extraction of essential oil from hyptissuaveolens leaves
Hyptissuaveolens(1kg) leaves were washed and placed in round bottom flasks of the hydro-distillation set up with 1liter distilled water. The round bottom flask was then heated on heating mantle for 2 hrs. The essential oil evaporated due to the heat, it was cooled and collected as distillate (mixture of essential oil and water). This was transferred to a glass separating funnel and the essential oil separated from the water based on density. Percentage yield of the essential oil extracted was then calculated using this formula;
Characterization of essential oil
Gas chromatography-mass spectroscopy (GC-MS)
The essential oil was subjected to GC-MS analysis on a GC-MS instrument with Elite – DB- 5M column and the GC-MS solution version 2.53 software. Initially oven temperature was maintained at 60oC for 3.50 minutes, and the temperature was gradually increased up to 250oC at 10.0/24.0 min and 4.0 μl of sample was injected for analysis. Helium gas 99.995% of purity was used as a carrier gas as well as anfluent. The flow rate of helium gas was set to 0.99 ml/min. The sample injector temperature was maintained at 250ºC and the split ratio is 40 throughout the experiment periods. The ionization mass spectroscopic analysis was done with 70 eV. The mass spectra were recorded for the mass range 50-600 m/z for about 24 minutes. Identification of components was based on comparison of their mass spectra. As the compounds separated on elution through the column they were detected in electronic signals. As individual compounds eluted from the Gas chromatographic column, they entered the electron ionization detector where they were bombarded with a stream of electrons causing them to break apart into fragments. The fragments were actually charged ions with a certain mass. The m/z ratio obtained was calibrated from the graph obtained which was called as the mass spectrum graph which is the fingerprint of the molecule. The identification of compounds was based on the comparisons of their mass spectra with NIST Library 2008 WILEY8, FAME. Quantitative determinations were made by relating respective peak areas to TIC areas from the GC-MS (Purushoth, et. al., 2013; GCMS analysis, NARICT Zaria).
Fourier transform infra-red (ftir) spectroscopic analysis
FTIR relies on the fact that the most molecules absorb light in the infra-red region of the electromagnetic spectrum. This absorption corresponds specifically to the bonds present in the molecule. The frequency ranges are measured as wave numbers typically over the range 4000 – 600 cm-1. The background emission spectrum of the IR source is first recorded, followed by the emission spectrum of the IR source with the sample in place. The ratio of the sample spectrum to the background spectrum is directly related to the sample's absorption spectrum. The resultant absorption spectrum from the bond natural vibration frequencies indicates the presence of various chemical bonds and functional groups present in the sample.
Determination of physicochemical properties
Determination of iodine value
For the determination of Iodine Value, Firestone’s method, (1994) was used. The essential oil (0.35g) was weighed accurately into a 250 ml Erlenmeyer flask and 20 ml carbon tetrachloride (CCL4) solution was added. Another 20 ml of carbon tetrachloride was placed into another flask to serve as blank. Firestone’s (25 ml) reagent was added to both flasks. The two flasks were stoppered; the contents were mixed well by swirling and stored in a dark place at 25 ± 5°C for 30 minutes. At the end of 30 minutes, 10 ml of 30% potassium iodide solution and 100 ml of purified water were added to both sample solutions. These were titrated immediately with standard 0.1 N thiosulfate solutions until the yellow colour almost disappears. One(1) ml of starch solution which served as indicator was added and titrated drop-wise with vigorous swirling till the disappearance of the blue starch-iodine colour. The blank was titrated in the same manner.
The equation below was used to calculate the iodine value.
12.69 is a constant, Blt = result obtained from the blank, St = result gotten from the test and M = molarity.
Determination of saponification value
Saponification Value determination was carried out according to the method described in Lubrizol.15,16 One (1)gm of the essential oil was weighed and transferred into a round bottomed flask. To the content of the round bottom flasks, 20 ml of 0.5 N alcoholic KOH solutions was added. Another flask was set up without the essential oil which served as blank. The two round bottomed flasks were refluxed for 1 hour. After the refluxing, both the round bottomed flasks were allowed to cool. Titration was carried out using 0.5 N HCl with phenolphthalein indicator till the disappearance of pink colour, which indicated the end point. The saponification value was calculated using the formula below.
This was done in triplet.
B = end point value for the blank, S = end point value for test, 280.5 = constant and 0.1 = molarity.
Determination of acids value
This was done using the method described in Lubrizol.15,16 The test substance, which is the essential oil from the leaves of Hyptissuaveolens(0.5 grams),was taken into a 250 ml Erlenmeyer flask. Ethyl alcohol (50 ml) was added to the Erlenmeyer flask, the solution was then mixed by gently heating the flask in a water bath until all the essential oil was dissolved. Few drops of phenolphthalein indicator were added to a 20 ml 0.1N KOH aqueous solution in a beaker. This mixture (phenolphthalein indicator and 0.1N KOH) was then titrated against the solution in the Erlenmeyer flask till the colour less solution turns to pink. The amount of KOH used was recorded as the acid value. The above was repeated three times and average result taken.
The equation, Acid value = was used to calculate the acid value.
M stands for molarity, St for test result and 56.10 a constant.
Determination of peroxide value
Peroxide value determination was done as described in Lubrizol 2006. The essential oil (2.00g) was measured into a 100 ml glass stoppered Erlenmeyer flask. Acetic acid - chloroform solution (12 ml) was measured and added to the essential oil. The flask was swirled until the sample is completely dissolved this was done carefully by warming the flask on a hot plate. Saturated potassium iodide solution (0.2 ml) having a concentration of 0.01mol was then added to the flask, stoppered and contents swirled for 1 minute exactly. Twelve (12) mls of deionized water was added immediately and shaken vigorously, iodine formed in the chloroform layer was liberated. One (1) mL starch solution (as an indicator) was added to 50 mL 0.1N sodium thiosulfate solution in a beaker; this mixture is emptied into a burette and titrated against the contents of the Erlenmeyer flask until the disappearance of blue gray colour that was in the aqueous upper layer. The whole procedure was repeated without the essential oil which served as the blank for the experiment. The above was done in triplet. Calculation was done using the formula;
V1 is the end point from test, v0 is end point from blank, M is molarity and 1000 a constant.
Determination of free fatty acids value
The determination of free fatty acids value according to the method described in Lubrizol. Free Fatty Acids
The above formula was used in calculating the free fatty acid value in percentage.Where1.99 is a constant.
Determination of ester value
This is described in Lubrizol 2010 as, Ester value = saponification value - acids value.
Percentage yield of extracted essential oil from hyptissuaveolens leaves
The quantity of essential oil obtained from the three (3) independent extractions using 1 kg in each case of leaves of Hyptissuaveolens is presented in Table 1. An average quantity of 0.4-0.5 mls was extracted and the average yield was 0.1%. The oil is a clear liquid with physical appearance of pale yellow.
Weight of leaves used |
Quantity of oil obtained |
One (1) kilogram |
0.5 mls |
One (1) kilogram |
0.6 mls |
One (1) kilogram |
0.4 mls |
Average quantity extracted |
0.5 mls |
Percentage yield of essential oil extracted |
0.1 |
Table 1 Extracted Essential oil from Hyptissuaveolens leaves
Gas chromatography- mass spectroscopy (GC-MS)
Characterization of the essential oil from hyptis suaveolens leaves by GC-MS
The mass spectra of Hyptis suaveolens poit obtained from GC-MS analysis revealed that the essential oil from the leaves of this plant contains twenty (20) compounds (Table 2). The peaks of the compounds are in Figure 1.
|
Peak Num |
Retention time |
Area (%) |
Compound Name |
Molecular weight |
Molecular formulae |
Structure |
S.I |
|
1 |
6.014 |
1.81 |
1S-alpha-Pinene |
136 |
C10H16 |
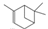 |
97 |
|
2 |
6.646 |
6.82 |
Bicyclo[3.1.0]hexane |
136 |
C10H16 |
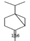 |
93 |
|
3 |
6.735 |
3.73 |
Beta-Pinene |
136 |
C10H16 |
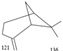 |
94 |
|
4 |
6.877 |
5.36 |
Octen-3-ol |
128 |
C8H100 |
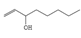 |
95 |
|
5 |
7.147 |
2.03 |
Alpha- Phellandrene |
136 |
C10H10 |
 |
93 |
|
6 |
7.626 |
31.12 |
Eucalyptol |
156 |
C10H180 |
 |
93 |
|
7 |
7.997 |
1.47 |
Bicyclo[3.1.1]hept-2-ene |
136 |
C10H16 |
 |
93 |
|
8 |
8.478 |
4.52 |
Cyclohexene,4-methyl-3 -(1-methylethylidene |
136 |
C10H16 |
 |
93 |
|
9 |
8.586 |
2.08 |
Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl |
152 |
C10H160 |
 |
95 |
|
10 |
10.071 |
3.55 |
3-Cyclohexen-1-ol,4- methyl-1-(1-methylethyl) |
154 |
C10H100 |
 |
93 |
|
11 |
12.766 |
1.32 |
Copaene |
204 |
C15H24 |
 |
87 |
|
12 |
12.592 |
2.79 |
Cyclohexane,1-ethenyl-1-methyl-2,4-bis(1-methylethenyl) |
204 |
C10H15 |
 |
89 |
|
13 |
13.483 |
17.3 |
Bicyclo[7.2.0]undec-4-ene4,11,11-trimethyl-8- methylene,[IR(IR*,4Z,9S*)] |
204 |
C15H24 |
 |
90 |
|
14 |
14.386 |
6.81 |
1,6-Cyclodecadiene,1- methyl-5-methylene-8-(1- methylethyl),[s-(E,E)] |
204 |
C15H24 |
 |
87 |
|
15 |
14.617 |
2.57 |
gamma-Elemene |
204 |
C15H24 |
 |
91 |
|
16 |
16.362 |
1.13 |
1H-3a,7 Methanoazulene, octahydro-1,4,9,9- tetramthyl |
206 |
C15H26 |
 |
87 |
|
17 |
18.149 |
1.3 |
Bergamotol,Z-alpha-trans |
220 |
C24H24O |
 |
91 |
|
18 |
22.223 |
2.68 |
Phenanthrene,7-ethenyl- 1,2,3,4,4a,4b,5,6,7,8,8a,9-dodecahydro-1,1,4b, tetramethyl-,[4aS-(4a.alpha.,4b. beta.,7.alpha.,8a.alpha.)] |
272 |
C20H32 |
 |
79 |
|
19 |
22.684 |
1.02 |
7-Isopropyl-1,1,4a trimethyl1,2,3,4,4a,9,10,10a-0ctahydrophenanthrene |
270 |
C20H30 |
 |
76 |
|
20 |
22.966 |
0.58 |
1,3,6,10- Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1- methylethyl) |
272 |
C20H32 |
|
80 |
Table 2 Components of the essential oil from Hyptissuaveolens by GC-MS
The most abundant compound is Eucalyptol with peak area of 31.12% and retention time of 7.626. This is followed by Bicyclo[7.2.0]undec-4-ene4,11,11-trimethyl-8-methylene, with a peak area of 17.30% and retention time of 13.483, while 1,3,6,10-Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl) is the least abundant compound with a peak of 0.58% and a retention time of 22.966. From the entire compounds identified by GC-MS, the percentage similarity to that in the mass spectra data base was found ranging between 80-97%. Table 3 showed the break-down of the components in the essential oil from Hyptis suaveolens. Terpenes has the highest percentage (46.97) followed by alcohol with 43.41% and other hydrocarbons 9.61%.
Compounds present in the essential oil of Hyptissuaveolensleaves |
Amount in percentage |
Terpenes |
46.97 |
Alcohol |
43.41 |
Other |
9.61 |
Table 3 Breakdown of components of the essential oil from Hyptissuaveolens by GC-MS
Fourier transform infra-red spectroscopy (FTIR)
Characterization of the bioactive fraction by FTIR
Characterization of the bioactive fraction of essential oils from Hyptissuaveolens(L. Poits) was carried out to determine the types of functional group(s) in the essential oils of Hyptissuaveolens fraction. The spectra reveal the presence of fifteen (15) functional groups (Table 4). The peak at 448.46, indicate the presence of a C-I stretch bond Alkyl halides of strong intensity and its derivatives group present. The peak at 1078.24, indicate the presence of a C-O stretch bond Alcohols of medium strong intensity and its derivatives. The peak at 1160.22 and 1299.1, indicate the presence of O=C-O-C stretch bond esters (aliphatic and aromatic compounds respectively) both having strong, very strong intensity. At the peak 1231.59, there is the presence of =C-O-C stretch bond ether of medium strong intensity. C-H bend is present at the peak 1450.52, Alkenes and Alkyls of strong intensity. At peak 1644.37, an indication of C=O stretch Amides is present with strong broad intensity. At the peaks 2943.47 and 3431.48, an indication of O-H stretch Carboxylic Acids are present with strong, broad intensity. At peaks 3736.24 and 3841.36, an indication of N-H symmetric bond of weak intensity is present. (Figure 2).
Peak num. |
Absorbance(nm) |
Class of compd |
Functional group |
Intensity |
1 |
448.46 |
Alkyl halides (R-I) |
C-I stretch |
S |
4 |
1078.24 |
Alcohols(R-CH2- OH) ˈ |
C-O Stretch |
m-s |
5 |
1160.22 |
Esters(aliphatic) |
O=C-O-C stretch |
s, vs |
6 |
1231.59 |
Ethers(Ar-O-R) |
=C-O-C stretch |
m-s |
7 |
1299.1 |
Esters(aromatic) |
O=C-O-C stretch |
s, vs |
8 |
1450.52 |
Alkenes and Alkyls |
C-H bend |
S |
9 |
1644.37 |
Amides(R-C(O)-NH- R) |
C=O stretch |
s, broad |
12 |
2943.47 |
Carboxylic, Acids(R- C(O)-OH) |
O-H stretch |
s, broad |
13 |
3431.48 |
Carboxylic Acids(R- |
O-H stretch |
s, broad |
14 |
3736.24 |
Amines(R-NH2) |
N-H symmetric |
W |
15 |
3841.36 |
Amines(R-NH2) |
N-H symmetric |
W |
Table 4 Functional groups of the components of essential oil from Hyptissuaveolens by FTIR
Footnote:-vs-very strong, s- strong, m-medium, w-weak.
Physicochemical indices of essential oil from Hyptissuaveolens leaves
Essential oil from Hyptissuaveolens leaves can be identified using its physicochemical properties (Table 5)
Physicochemical Tests |
Composition |
Peroxide Value (mg/kg) |
40.00 +0.02 |
Iodine Value (g/100g) |
23.59+0.12 |
Saponification Value (mgKOH/g) |
100.18+0.80 |
Acid Value (mgKOH/g) |
3.37+0.01 |
Ester (mg/g) |
96.81 |
Free Fatty Acid Value (%) |
1.69 |
Table 5 Physicochemical analysis of essential oil from Hyptissuaveolens leaves
The clear liquid oil with physical appearance of pale yellow is same with all previous works. Essential oil yield of about 0.4-0.6ml/kg (equivalent to 0.1%) got in this studies is same with the work of Raizada17 who got a percentage yield of 0.1 from the same plant, never the less it is low compared with the work of the following researchers; Iwalokun et al.,18 a percentage yield of 0.31 and Okonogi et al.,19 percentage yield of 0.21; The difference in percentage yield could be due to the moisture level of the leaves and the chemotypic profile of the Hyptissuaveolens strains analysed. Latitude, altitude, soil composition, climate and genetic composition are factors that have been implicated for chemotype variations in Hyptissuaveolens and other species of Hyptis as well as other aromatic herbs belonging to the Lamiaceae family. Various bioactive compounds were recovered from the essential oil samples of this chemotypes with variations in yield, composition and pharmacological effects Mandal et al.,20 got the following percentage yield from the same plant using several solvent which are Steam distillation (yield: 0.24%), petroleum ether extract (yield: 1.6%) and ethanol extract (yield: 2.64%); Bachheti et al.,21 got a percentage yield of 17.44 from seed oil and not leave oil; Shenoy et al.,22 reported percentage yield of 4.78% for Petroleum ether, 8.52% for Solvent ether, 3.30% for Chloroform, 5.48% for Alcohol and 15.22% for Chloroform water; Gavani et al.,23 got a percentage yield of 4.86% methanol extract. The difference in these studies and the present work could be due to the different solvents used and in one case different part of the plant, apart from the above mentioned factors.
The result of GC-MS revealed that terpenes are the most abundant component in the essential oil with the highest percentage of 46.97, this is followed by alcohol which consituent 43.41 other hydrocarbons were 9.61 percents (%) (Table 2). Terpenes are known to repel insects.24–26
The presence of the compounds found in this work corresponds with the works of Moreira et al.,2 which showed that the following are present in the essential oil of Hyptissuaveolens; β-pinene (6.55%), 1-octen-3-ol (0.28%), eucaliptol (47.64)%, gama-ellemene (8.15%), etc and Umedum et al.,10 who also showed that, Cyclohexane (0.47%), α-pinene (2.04%), β-pinene (6.72%), 1-Octen-3-ol (2.42%), β-Elemene (0.6%), Bergamotol (0.64%), Phenanthrene (0.72%), etc. all of which are terpenes.
The most abundant compound of the essential oil in this work is Eucalyptol with peak area of 31.12% and retention time of 7.626, This corresponds with the work of Moreira et al.,2 these authors revealed that Eucalyptol, gama-ellemene and β-pinene are among the most abundant components (47.64 %, 8.15% and 6.55 % respectively). The work of Franz et al.,27 shows that Phellandrene is abundant in the essential oil of Hyptissuaveolens which is also present in this study. Vongsombath showed that α-pinene is a major component of Hyptissuaveolens which correspond with this study. The work of Noudogbessi et al.,28 is also in perfect agreement with this study. Little differences in the percentages obtained may be due to the solvents used for the extraction,moisture level of the leaves and the chemotypic profile of the Hyptissuaveolens strains analysed.
The next abundant component of the essential oil in this study is Bicyclo[7.2.0]undec-4-ene4,11,11-trimethyl-8-methylenewith a peak area of 17.30% and retention time of 13.483, while 1,3,6,10-Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl) is the least abundant compound with a peak of 0.58% and a retention time of 22.966. From the entire compound identified by GC-MS the percentage similarity to that in the mass spectra data base was found ranging between 80% and 97%.
Characterization of the bioactive fraction of essential oils from Hyptissuaveolens(L. Poits) was carried out to determine the types of functional group(s) in the essential oils of Hyptissuaveolensfraction. The spectra reveal the presence of fifteen (15) functional groups as shown in Table 4, which indicates the presence of the following class of compounds; esters, ethers, alkenes, alkyls, alcohols, amines, amides, alkyl halides and carboxylic acids. Esters are used for making surfactants such as soap, detergent etc. They are also used for making perfumes because of their sweet smell, unsaturated esters have stronger odour than the saturated ones. Ethers have pleasant smell that makes them suitable for use in the preparation of perfume, soap, anaesthesia etc.
Iodine value is determine by the degree of unsaturation of oils and fats and hence tendency of a fatty oil to absorboxygen. The higher the iodine value the higher the degree of unsaturation and the likelihood for the oil to go rancid. Oils with low iodine value are good for soap-making they are referred to as non-drying oil (STANDS4 LLC, 2015).The higher the iodine value the more reactive, less stable, softer, and more susceptible to oxidation and rancidification is the oil, fat, or wax. Iodine value of 23.59g/100g obtained from this study indicates that the oil can be used for soap-making. Oils with higher iodine values are not good for soap-making.
Saponification values are highly significant to consider in soap making. It is important that the saponification value is high enough (not too much alkali) but to contain sufficient soapiness, low saponification value means small fatty acid salts which may not be sufficient enough to remove or saponify the fat or oil due to less soapiness.Saponification value of 100.18mgKOH/g was obtained from this study which is high enough (good) for soap-making. The result is similar to that of Umedum et al.,10 and Bachheti et al.,21 who both obtained saponification value of over 100mgKOH/g.
Acid value is the amount of KOH or NaOH required in milligram to neutralize one gram of oil. Oils with low acid value are better in making soap. The acid value of 3.37mgKOH/g obtained from this study make it good oil that can be used on the skin. This value corresponds with the work of Umedum et al.,10 who recorded 3.3mgKOH/g and Bachheti et al.,21 who also recorded an acid value of 3.3mgKOH/g.
Peroxide value shows how fats and oil can undergo autoxidation; it shows the extent of the spoilage of oil. Peroxides are intermediates in autoxidation of oil; this shows that high peroxide value shows the extent of oil spoilage. A peroxide value of 40 meq/kg shows that the oil is good for use either on the body or for consumption.
Free Fatty Acids value (%) is a shelf life indicator test for hydrolytic rancidity, free fatty acid value (%) of 1.69 is ideal for use either on body or consumption. Esters with low molecular weights, such asethylacetate, are usually volatile fragrant liquids; fats are solid esters with pleasantaromas. An ester value of 96.81mg/g was obtained in this study (The American Heritage Science Dictionary, 2002). There is no much information on ester value. Looking at the physicochemical properties of the essential oil from Hyptissuaveolens leaves, the oil can be formulated into cream19 or perfumes to be used to keep mosquitoes away.29–32
Percentage yield of 0.1 is averagely sufficient using hydro-distillation. Characterization of the essential oil done through GC-MS revealed the presence of terpenes to be the most abundant component. FTIR analysis revealed the presences of functional groups such as esters, ethers, carboxylic acids etc all of which are useful in the making of soap, perfume, anaesthesia and several other things. Characterization of the oil through determination of physicochemical properties revealed that the iodine, acid, saponification, peroxide, ester and free fatty acids values suggest that the oil can be formulated into cream or perfume to be used directly on the skin to repel mosquitoes considering its friendly nature both to the skin and the environment.
None.
The authors declare there are no conflicts of interest.

©2020 Joseph, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.