International Journal of
eISSN: 2573-2889


Research Article Volume 4 Issue 2
Department of Biochemistry, Michael Okpara University of Agriculture Umudike, Nigeria
Correspondence: Egbuonu Anthony Cemaluk C, Department of Biochemistry, College of Natural Sciences, Michael Okpara University of Agriculture Umudike, Nigeria, Tel +23480-3636- 6565
Received: January 26, 2019 | Published: April 2, 2019
Citation: Obi E, Egbuonu ACC. Changes in the liver histomorphology, catalase and glutathione peroxidase activity in the serum and liver homogenate of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine. Int J Mol Biol Open Access. 2019;4(2):67?73. DOI: 10.15406/ijmboa.2019.04.00099
Artemether-lumefantrine (AL), an artemisinin-based anti-malarial, and monosodium glutamate (MSG), a flavor enhancer, respectively mediated oxidative stress with potential interactive effects in non-malarial-infected-hosts. Thus, changes in the liver histomorphology, catalase (CAT) and glutathione peroxidase (GPx) activity in the serum and liver homogenate of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine were investigated. Thirty rats randomly sectioned into six (n = 5) were exposed to feed and water in addition to nothing (Group A, control), therapeutic dose of AL (Group B), overdose (therapeutic dose ×5) of AL (Group C), MSG, at 8000 mg/kg body weight (Group D), therapeutic dose of AL plus MSG (Group E) and overdose of AL plus MSG (Group F). Rats exposed to MSG either alone or together with overdose of AL had overriding reduction in the serum and liver homogenate CAT and Gpx activities, computed Gpx:CAT and CAT:Gpx ratios, and severe histological changes compared to the control and others, suggesting significant adverse effect of overdose of AL together with MSG on the rats’ liver morphology and in the serum and liver homogenate CAT and GPx activities. Thus, exposure of normal rats to, in particular, overdose of AL together with MSG, exerted significant adverse influence on the rats’ liver by way of compromised liver morphology and antioxidant capacity. It was admonished that non-malaria parasite infected rats should not be exposed to AL, irrespective of dose, either alone or together with intoxicating dose of MSG. The possible implications of this study in humans evoke further studies, hence are recommended.
Keywords: artemesinin-based, oxidative stress, therapeutic dose, liver morphology, antioxidant capacity
AL, artemether-lumefantrine; MSG, monosodium glutamate; CAT, catalase; GPx, glutathione peroxidase; ACTs, artemisinin-based combination therapies; WHO, world health organization; CYP450, cytochrome p450; H2O2, hydrogen peroxide; IU/L, international unit per litre; GSH, glutathione; NADPH, reduced nicotinamide adenine dicnucleotide phosphate; NADP+, oxidized nicotinamide adenine dinucleotide phosphate; NM, nanometer; ANOVA, analysis of variance; SPSS, statistical package for social sciences; SEM, standard error of mean
Artemisinin-based combination therapies (ACTs), a first line anti-malarial in drug resistant malarial parasite endemic sub-Saharan African countries, including Nigeria.1–3 could, aside delaying drug resistance,2,4 exert early parasitological response while the partner drug could prevent recrudescence by slowly eliminating the remaining malaria parasites.4,5 The commonly used artemether-lumefantrine, as the other artemesisnin-based combination therapies, acts by generating free radicals (pro-oxidants) that kill the malaria parasites.6–8 Monosodium glutamate (MSG), a flavor enhancer with unique taste widely used in canned foods,9–11 could induce toxic influences and oxidative stress in animals.12–17 In particular, oxidative stress which could cause even base damage and strand breaks in DNA,18 is implicated in the etiology of many diseases.19–22 Thus, the possible interactive effects of MSG with artemether-lumefantrine on the antioxidant response status of animals may be significant, particularly in non-malaria-parasite-infested host. This may be notable in the liver which serves as the major detoxification organ,23,24 using cytochrome P450 (CYP450), glutathione peroxidase, and catalase.24,25
Further to the above, as an over the counter drug a possibility of abuse of AL exists. With reported self-medication with antimalarials,26 possibility of overdose and even prolonged intake of AL exists. Also, possibility of co-intake of artemether-lumefantrine with monosodium glutamate-flavour enhanced fast foods, even at an overdose and in absence of malaria infection, exits. That notwithstanding, there is paucity in literature as regards possible effect on the antioxidant status following use or abuse of AL either alone or in combination with intoxicating dose of MSG particularly in absence of malaria parasite infection. These warranted this study aimed at investigating changes in the liver histomorphology, catalase and glutathione peroxidase activity in the serum and liver homogenate of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine. Altered catalase and glutathione peroxidase activity indicated induction of oxidative stress while the changes in organ histology could confirm compromised organ function.27–29
The artemether-lumefantrine (20:120mg) combination used in this study was procured from a recognized medical representative in Umuaha, Abia State, Nigeria. Monosodium glutamate (99 % purity) was purchased from Umuahia market, Abia state, Nigeria. Thirty (30) male Wistar rats weighing 89-183g were bought from a commercial breeder at Nsukka, Enugu state Nigeria and transported in a well ventilated metal cages to the animal house of the department of biochemistry, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria. All animals were allowed a 14-day period of acclimatization before commencement of the experiment. The rats were randomly sectioned into six (6) groups of five rats each and respectively exposed freely to feed (vital feed pellets) and portable tap water in addition to nothing (Group A, control), therapeutic dose of AL (Group B), overdose of AL (Group C), MSG (Group D), therapeutic dose of AL plus MSG (Group E) and overdose of AL plus MSG (Group F). The exposure was oral using a gavage. Artemether-lumefantrine overdose was calculated as therapeutic dose for 70 kg man multiplied by 5. The administration of artemether-lumefantrine was twice each day after 8hrs interval, in line with the prescription format of its dosage in respect to a 70 kg man given in volume (prepared by dissolving adult dose (4 tablets) of artemether-lumefantrine in 100 ml of distilled water) corresponding to the rats weight according to manufacturer’s instruction. In brief, the first and second dose exposures were eight hours apart while the third dose exposure was 24 hours after the first or 16 hours after the second whereas the remaining doses (fourth, fifth and sixth) were given 12 hours apart. Rats intoxication with MSG was achieved at 8000 mg/kg body weight and by daily exposure for 7 days according to Mariyamma et al.,13 as supported by other studies.30–33
Ethical consideration
Throughout the experimentation (acclimatization and exposure periods), all rats were housed at 25oC in stainless steel cages under normal daylight/day cycle and humid tropical conditions. The rats were allowed free access to rat feed (vital feed pellets) and tap water and were conditioned throughout the process according to the guidelines approved by the department official committee on animal use, Michael Okpara University of Agriculture, Umudike on handling of experimental rats.
Sacrifice and sample collection
After 7 days, the rats were sacrificed the next day after overnight fast by ocular puncture and the blood sample of the respective rats was collected into a clean non-anticoagulated polystyrene tube, allowed to clot and centrifuged at 3000rpm for 5 minutes and the serum collected and stored in a refrigerator until used. The liver of the respective rat was excised and the section for the histological study was placed in a tube containing formalin. The remaining liver section was rinsed in iced-cold sucrose, and a 10 % w/v homogenate was prepared from it using 0.15 M KCl as buffer to obtain the supernatant sample after centrifugation.31
Measurement of oxidative stress markers
Serum and liver homogenate activity of the catalase was determined by the method as described by Sinha34 based on the principle that dichromate in acetic acid was reduced to chromic acetate when heated in presence of hydrogen peroxide (H2O2) with the formation of per chromic acid as an unstable intermediate. In brief, to 0.9ml of phosphate, 0.1ml of serum (or liver homogenate) and 0.4ml of H2O2 were added. The reaction was initiated by adding 2ml of dichromate acetic acid mixture. The tubes were kept in a boiling water bath for 10 minutes, cooled and the colour developed was read at 530nm at intervals of 30 minutes for 2 hrs. Standards in the concentration range of 20 – 100 micromoles were processed for the test. The catalase activity was calculated as micromoles of H2O2 utilised/second expressed as international unit per litre (IU/L).
Serum and liver homogenate glutathione peroxidase activity was determined by the method of Paglia et al.,35 based on the principle that glutathione peroxidase (Gpx) catalyzes the oxidation of glutathione (GSH). In brief, a known volume, 0.05ml of serum (or liver homogemate) was diluted with 2ml of diluting reagent. To 50μl of either the diluted sample or blank was mixed with 1ml of reagent 1 (glutathione+glutathione reductase+NADPH) and reagent 2 (cumene hydroperoxide) respectively. The initial absorbance of the test or the blank was respectively read after 1 min and the timer started simultaneously. In the presence of glutathione reductase and NADPH the oxidized glutathione is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340nm was measured by reading absorbencies after 1 and 2 minutes intervals. Glutathione peroxidase activity was calculated from the formulae below:
GPx (IU/L) = 8412 x ΔA 340nm/minute.
Histopathological examination
The respective liver section of the rats collected for histopathological studies was prepared as previously reported.36 In brief, the respective liver sections were fixed in 10% phosphate buffered formalin for 48 hours, trimmed, dehydrated in 4 grades of alcohol (70%, 80%, 90% and 100% or absolute alcohol), cleared in 3 grades of xylene and embedded in molten wax. On solidifying, the blocks were sectioned into 5μm thickness with a rotary microtome, floated in water bathe and incubated at 60˚C for 30 minutes. The 5μm thick kidney sections were subsequently cleared in 3 grades of xylene and rehydrated in 3 grades of alcohol (90%, 80% and 70%). The sections were then stained with Hematoxylin for 15 minutes and blued (stained blue) with ammonium chloride. Differentiation was done with 1% acid alcohol before counterstaining with Eosin. Permanent mounts were made on degreased glass slides using a permanent DPX mountant (Model 44581, Sigma-Aldrich, United Kingdom). The prepared slides were examined with a Motic™ compound light microscope at various magnifications (×4, ×10 and ×40) of the objective lenses. The photomicrographs were taken using a Motic™ 9.0 megapixels microscope camera at x400 magnification.
Calculation of diagnostic ratios and change relative to groups
Diagnostic ratios were calculated from the result of corresponding parameters as obtained in this study. The calculation of change relative to any group was as developed and severally used.28,37–39
Change relative to either control or MSG group was calculated using the relation:
Change relative to K (%) = (V−K)/K×100
Where, K represents the constant group hence constant value and V represents the variable group hence variable values.
Statistical analysis
The data were subjected to one way analysis of variance (ANOVA) using Statistical package for social sciences (SPSS) version 20.0. Results were expressed as mean±standard error of mean (SEM). Difference was accepted as significant at p< 0.05.
The result as shown on Table 1 revealed that the serum catalase activity of rats in the other groups as compared to rats in the control was lower (p<0.05). The decrease in serum catalase activity relative to the control group was highest in the MSG only group (58.38%) followed by that in rats exposed to overdose of artemether-lumefantrine combination (AL) alone (46.70%) and least in the therapeutic dose of AL (11.68%). However, the increase relative to the MSG group aside the control (140.24%) was highest in the therapeutic dose of AL (112.20%).
|
Groups |
CAT (IU/L) |
Change relative to the |
Change relative to MSG group (%) |
|
A: Control (feed + water only) |
3.94 + 0.13 |
0 |
140.24 |
|
B: Therapeutic dose of AL |
3.48 + 0.13 |
− 11.68 |
112.2 |
|
C: Overdose of AL |
2.10 + 0.09 |
− 46.70 |
28.05 |
|
D: MSG (8000 mgkg-1bwt) |
1.64 + 0.15 |
− 58.38 |
0 |
|
E: Therapeutic dose of AL + MSG |
3.37 + 0.13 |
− 14.47 |
105.49 |
|
F: Overdose of AL + MSG |
2.35 + 0.15 |
− 40.36 |
43.29 |
Table 1 Changes in the catalase activity in the serum of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine
Values are mean±SEM for n=5. + denotes higher by; − denotes lower by. Difference considered statistically significant at p<0.05
The result as shown on Table 2 revealed that rats exposed to MSG alone had lower (p<0.05) liver homogenate catalase activity compared to the control. However, the liver catalase activity of rats in the therapeutic dose of AL, overdose of AL and overdose of AL+MSG were higher (p<0.05) than that of the rats in the control. The increase in catalase activity relative to the control was highest in the therapeutic dose of AL treated group of rats (61.99%) followed by that of rats in the overdose of AL (39.18%). The increase in liver catalase activity relative to the MSG treated group was highest in the rats exposed to the therapeutic dose of AL alone (134.75%).
|
Groups |
CAT (IU/L) |
Change relative to the |
Change relative to MSG group (%) |
|
A: Control (feed + water only) |
1.71 + 0.14 |
0 |
44.92 |
|
B: Therapeutic dose of AL |
2.77 + 0.33 |
61.99 |
134.75 |
|
C: Overdose of AL |
2.38 + 0.16 |
39.18 |
101.69 |
|
D: MSG (8000 mgkg-1bwt) |
1.18 + 0.09 |
− 30.99 |
0 |
|
E: Therapeutic dose of AL + MSG |
1.65 + 0.20 |
− 3.51 |
39.83 |
|
F: Overdose of AL + MSG |
1.93 + 0.15 |
12.87 |
63.56 |
Table 2 Changes in the catalase activity in the liver homogenate of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine
Values are mean±SEM for n=5. + denotes higher by; − denotes lower by. Difference considered statistically significant at p<0.05
The result as shown on Table 3 revealed that rats exposed to MSG (either alone or together with overdose of AL) had overriding reduction in the serum Gpx activity of the rats compared to the control and the other test groups. The decrease in Gpx activity relative to the control was highest in MSG treated group of rats (64.34%). The increase in serum Gpx activity relative to the MSG treated group was highest in the rats exposed to overdose of AL+MSG (349.10).
|
Groups |
GPx (IU/L) |
Change relative to the |
Change relative to MSG group (%) |
|
A: Control (feed + water only) |
4.60 + 0.16 |
0 |
175.45 |
|
B: Therapeutic dose of AL |
3.82 + 0.17 |
− 16.96 |
128.74 |
|
C: Overdose of AL |
4.62 + 0.98 |
0.43 |
176.65 |
|
D: MSG (8000 mgkg-1bwt) |
1.64 + 0.57 |
− 64.34 |
0 |
|
E: Therapeutic dose of AL + MSG |
3.66 + 0.16 |
− 20.43 |
119.16 |
|
F: Overdose of AL + MSG |
7.50 + 0.15 |
63.04 |
349.1 |
Table 3 Changes in the glutathione peroxidase (GPx) activity in the serum of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine
Values are mean±SEM for n=5+ denotes higher by; − denotes lower by. Difference considered statistically significant at p<0.05
The result as shown on Table 4 revealed that the Gpx activity in the liver homogenate of rats in the other groups as compared to rats in the control was lower (p<0.05). The decrease in liver Gpx activity relative to the control group was highest in the MSG only group (33.63) followed by that in rats exposed to overdose of AL+MSG (22.47) and least in the overdose of AL (1.74%). However, the increase relative to the MSG group aside the control (50.67) was highest in the overdose of AL treated group (48.05).
|
Groups |
GPx (IU/L) |
Change relative to the control (%) |
Change relative to MSG group (%) |
|
A: Control (feed + water only) |
20.07 + 2.34 |
0 |
50.67 |
|
B: Therapeutic dose of AL |
17.51 + 0.70 |
− 12.76 |
31.46 |
|
C: Overdose of AL |
19.72 + 0.71 |
− 1.74 |
48.05 |
|
D: MSG (8000 mgkg-1bwt) |
13.32 + 0.54 |
− 33.63 |
0 |
|
E: Therapeutic dose of AL + MSG |
15.77 + 0.24 |
− 21.43 |
18.39 |
|
F: Overdose of AL + MSG |
15.56 + 0.31 |
− 22.47 |
16.32 |
Table 4 Changes in the glutathione peroxidase (GPx) activity in the liver homogenate of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine
Values are mean±SEM for n=5+ denotes higher by; − denotes lower by. Difference considered statistically significant at p<0.05
The result as shown on Table 5 revealed overriding higher computed serum Gpx: CAT ratio but lower CAT: Gpx ratio in rats exposed to overdose of AL together with intoxicating dose of MSG compared with the other groups including the control and the MSG alone groups.
|
Groups |
CAT:GPx (GPx:CAT) |
Change relative to the control (%) |
Change relative to MSG group (%) |
|
A: Control (feed + water only) |
0.86 (1.17) |
0.00 (0.00) |
− 12.24 (+14.71) |
|
B: Therapeutic dose of AL |
0.91 (1.10) |
+ 5.81 (− 5.98) |
− 7.14 (+7.84) |
|
C: Overdose of AL |
0.45 (2.20) |
− 47.6 (+88.03) |
− 54.08 (+115.69) |
|
D: MSG(8000 mgkg-1bwt) |
0.98 (1.02) |
+ 13.95 (− 12.82) |
0.00 (0.00) |
|
E: Therapeutic dose of AL + MSG |
0.92 (1.09) |
+ 6.98 (− 6.84) |
− 6.12 (+6.86) |
|
F: Overdose of AL + MSG |
0.31 (3.19) |
− 63.95 (+ 172.65) |
− 68.37 (+212.75) |
Table 5 Changes in the catalase to glutathione peroxidase (CAT:GPx) and glutathione peroxidase to catalase (GPx:CAT) ratios in the serum of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine
Values are mean±SEM for n=5. + denotes higher by; − denotes lower by. Difference considered statistically significant at p<0.05
The result as shown on Table 6 revealed higher (p<0.05) computed liver CAT: Gpx ratio but lower (p<0.05) Gpx: CAT ratio in the liver homogenate of rat in the various groups compared to rats in the control and MSG groups. The observation relative to rats in either the control or the MSG group was highest in the group of rats exposed to the therapeutic dose of AL.
|
Groups |
CAT:GPX (GPX:CAT) |
Change relative to the control (%) |
Change relative to MSG group (%) |
|
A: Control (feed+water only) |
0.09 (11.74) |
0.00 (0.00) |
0.00 (3.99) |
|
B: Therapeutic dose of AL |
0.16 (6.32) |
77.78 (− 46.17) |
77.78 (− 44.02) |
|
C: Overdose of AL |
0.12 (8.29) |
33.33 (− 29.39) |
33.33 (− 26.57) |
|
D: MSG(8000 mgkg-1bwt) |
0.09 (11.29) |
0.00 (− 3.83) |
0.00 (0.00) |
|
E: Therapeutic dose of AL + MSG |
0.10 (9.56) |
11.11(− 18.57) |
11.11 (− 15.32) |
|
F: Overdose of AL + MSG |
0.12 (8.06) |
0.33(− 31.35) |
0.33 (− 28.61) |
Table 6 Changes in the catalase to glutathione peroxidase (CAT:GPx) and glutathione peroxidase to catalase (GPx:CAT) ratios in the liver homogenate of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine
Values are mean±SEM for n=5. + denotes higher by; − denotes lower by. Difference considered statistically significant at p<0.05
Changes in the liver histomorphology of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine
Compared to rats in the control and in the therapeutic dose groups, that showed normal hepatic lobules composed of normal hepatocytes arranged in interconnecting cords around the central vein in their histo-architecture (Figure 1 & Figure 2), mild to moderate changes including hepatocellular necrosis observed in the liver sections of the other groups (Figure 3–5) were moderate to severe in the group of rats exposed to overdose of AL together with MSG (Figure 6).
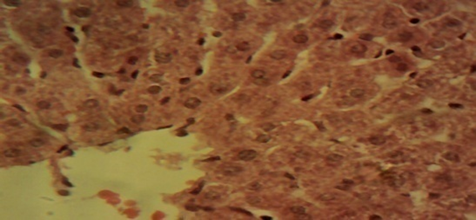
Figure 1 Photomicrograph of the liver sections from rats in the control showing normal hepatic lobules composed of normal hepatocytes arranged in interconnecting cords around the central vein (×400).
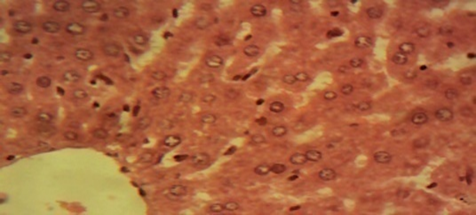
Figure 2 Photomicrograph of the liver of the therapeutic dose animal showing normal hepatic histomorphology for laboratory rodents (×400).
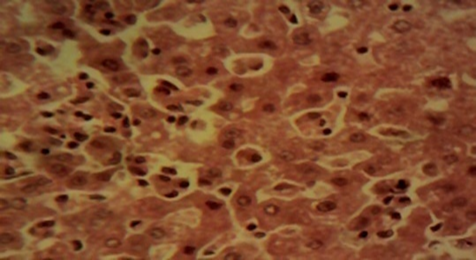
Figure 3 Photomicrograph of the liver of the overdose animal showing multifocal areas of hepatocellular necrosis (×400).
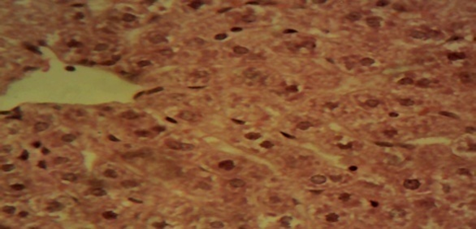
Figure 4 Photomicrograph of the liver of the MSG only animal showing mild to moderate diffuse, hepatocellular degeneration and necrosis involving all zones of the hepatic lobules (×400).
Artemether-lumefantrine (AL), an artemisinin-based anti-malarial, and monosodium glutamate (MSG), a flavor enhancer, respectively mediated oxidative stress with potential interactive effects in non-malarial-infected-hosts. Oxidative stress is fundamental in diseased conditions,36,40,41 thus, changes in the liver histomorphology, catalase (CAT) and glutathione peroxidase (GPx) activity in the serum and liver homogenate of normal and monosodium glutamate-intoxicated rats co-treated with artemether-lumefantrine were investigated. Monosodium glutamate intoxication of the rats was according to Mariyamma et al.13
Generally, catalase and glutathione peroxidase catalyze the conversion of hydrogen peroxide to water and gaseous oxygen.42 The result of the study on serum and liver homogenate catalase activity of rats revealed a decrease in activity which was, relative to the control, highest in the MSG only group followed by that in rats exposed to overdose of artemether-lumefantrine (AL) alone and least in the therapeutic dose of AL (Table 1 & Table 2), suggesting overriding induction of oxidative stress in the rats following exposure to either MSG or overdose of AL. Lower (p<0.05) CAT activity in MSG-exposed rats confirmed induction of oxidative stress in the MSG-treated rats.36 Decreased serum catalase activity43,44 and organ catalase activity8 indicated clinical signs of artemether-lumefantrine-induced toxicity. Artemether monotherapy also decreased liver catalase activity of the animals.45 Decreased CAT activity in the rats, as observed in this study, may have resulted from increased involvement of the enzyme in antioxidant defense response following MSG-induced oxidative stress.46 Apparently, the free radicals (oxidant) produced, particularly by either MSG or overdose of AL may have overwhelmed the rats’ antioxidant capacity via catalase enzyme activity which resulted to the depletion as observed.47,48
However, rats exposed to MSG (either alone or together with overdose of AL) had overriding reduction in the serum Gpx activity of the rats compared to the control and the other test groups, suggesting significant adverse effect of overdose of AL together with MSG on the serum and liver homogenate GPx activity of the rats. The observation on the GPx activity of the rats could be attributed to oxidant effect of MSG and artemether-lumafantrine that probably overwhelmed the antioxidant capacity via GPx activity of the rats in the reported groups. Glutathione peroxidase scavanges H2O249 and while further confirming MSG-induction of oxidative stress in the rats, the decreased GPx activity as observed could be resultant to the role of GPx as a second line of antioxidant defense mechanism following perhaps the overwhelmed antioxidant capacity of CAT, the first line of antioxidant defense mechanism. Decreased organ glutathione peroxidase activity8 indicated artemether-lumefantrine-related toxicity. The overriding higher (p<0.05) computed serum Gpx: CAT ratio but lower CAT:Gpx ratio (or higher (p<0.05) computed liver homogenate CAT:Gpx ratio but lower (p<0.05) Gpx:CAT ratio) in rats exposed to overdose of AL together with intoxicating dose of MSG compared with the other groups showed similar trend in apparent support of overriding adverse influence of MSG either alone or together with overdose of AL on the antioxidant capacity of the rats.
Toxic influence on the liver (including liver necrosis) of infected and uninfected rats following high doses of artemether has been reported.50 These connoted an overriding adverse influence of MSG together with overdose of AL on the hepatic histomorphology of rats in support of the result of this study obtained from the serum and liver homogenate of the rats.
Thus, exposure of normal rats to, in particular, overdose of AL together with MSG, exerted significant adverse influence on the rats’ liver by way of compromised liver morphology and antioxidant capacity. The study necessitated the admonition that non-malaria parasite infected rats should not be exposed to AL, irrespective of dose, either alone or together with intoxicating dose of MSG. The possible implications of the result of this study in humans evoke further studies, hence are recommended.
None.
The authors declare that no conflict of interest exists.

©2019 Obi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.