International Journal of
eISSN: 2573-2889


Research Article Volume 5 Issue 2
1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Jouf University, Saudi Arabia
2Department of haematology, Faculty of Medical laboratory science, University of Gezira, Sudan
3Central Medical laboratory, Gezira Hospital for Renal Diseases and Surgery, Sudan
4Department of Pathology, Faculty of medicine, Karary University, Sudan
5Department of Pathology, Faculty of medicine university of Gezira, Sudan
6Department of Medical Parasitology, Faculty of Medical laboratory science, University of Gezira, Sudan
Correspondence: Sanaa E Hussein, Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Jouf University, Skaka, Saudi Arabia (KSA)
Received: April 04, 2020 | Published: April 13, 2020
Citation: Hussein SE, Mohammed MA, haroon HM, et al. Association of FV leiden G 1690 A and prothrombin G 20210 in coagulation activation of sudanese sickle cell anemia patients. Int J Mol Biol Open Access. 2020;5(2):33?38. DOI: 10.15406/ijmboa.2020.05.00128
Background: Vascular thrombosis is an important pathophysiological aspect of sickle cell disease (SCD). This study aimed to detect the inherited risk factors FV Leiden G 1690 A and Prothrombin G 20210 A in sickle cell Anaemia patients. A total of 50blood samples collected from sickle cell disease patients in steady state. The control group consisted of 50 blood samples were collected from healthy people matched with age and gender of study subjects. All samples were investigated for PT, PTT, TT,using Semi automated STA4coagulometer. Level of D-dimer, TAT complex was estimated by ELIZA. Inherited mutations were detected by RFLP method using PCR machine TECHNE (TC-400). The study showed complete absence of FVL mutation (0) % in SCA patients in compare with prothrombin mutation which was found in (18.0%) while the frequency of two mutations in controls is (0%) Comparison of coagulation tests PT, PTT and TT between positive and negative Prothrombin G2021A mutation within the SCD patients, positive patients showed slight prolongation in mean PTT (39.1667s versus 36.6832s in negative patient paralleled with slightly higher in mean level TAT (10.57133 versus 4.94941 ng/ml) and mean level D-dimer (3541. Versus 2261.06 ng/ml). The study concluded that SCA patients exhibit chronic activation coagulation process even in steady state and moreenhanced in heterozygous prothrombin G1962A patients, cohort studies are needed to evaluate the clinical impact of prothrombin mutation on the frequency of vasoclusive crises and other complications.
Keywords: sickle cell disease, thrombophilia, FV Leiden G 1692 A, prothrombin G A 20210, Sudan
Sickle cell disease clinical manifestations arise from the tendency of the haemoglobin (HbS or sickle haemoglobin) to polymerize and deform red blood cells into the characteristic sickle shape. It is reported that African, Indian and Arab are the most affected ancestries by Sickle Cell Disease with more than 80% of 300 ,000 birth in sub-Saharan Africa annually.1–4 SCD has complex pathophysiology characterized by frequent painful episodes, hemolytic anemia, end-organ damage, and a shortened life span.5 The severity of illness in sickle cell anemia is highly variable and can vary even within families. Many children become symptomatic in infancy after 3 to 4 months of age. Chronic complications as thromboembolic events, stroke pain and other chronic complications occurs in older children, adolescents and adults.6 Persistent haemolysis and acute vasocclusive painful crises are the most common type of crisis with the prevalence of cerebrovascular accidents3 (4%) Acute pain episodes occurred in SCD patients are described as one of the most excruciating forms of pain affect the human beings.2,7 It is caused by microvascular occlusion which lead to stimulation of nociceptive nerve fibers, moreover, microvascular occlusion can result in ischemia, edema, necrosis, and organ damage.2 Pain can occur in the abdomen, bones, joints, or muscles. Young children often present with pain involving the hands or feet (the hand-foot syndrome or dactylitis), long bones and the abdomen are more common sites of pain in adults. Nonspecific cutaneous manifestations in the lower extremities as leg ulcers due to macro and micro thrombi start around 20 years of age and men are more likely to be affected than women.8 Arterial Ischemic stroke (AIS) is responsible of many neurological complications in children with SCD and subsequently neurologic morbidity worldwide.9
The term thrombophilia is used to describe the inherited or acquired disorders of the haemostatic mechanism that predispose to thrombosis.10 The heritable abnormalities that have been associated with increased risk of venous thrombosis include those due to reduction in anticoagulant function or deficiency of natural anticoagulants antithrombin, protein C or protein S and those associated with increased procoagulant activity, factor V Leiden and the G20210A prothrombin polymorphism (G2010 A) . FVL refers to a base change (from G to A at position 1691) in G1691A mutation in factor V gene 1q21-25 chromosome resulting in a substitution of glycine for arginine at position 506, called factor V Leiden, which makes the factor Va protein resistant to inactivation by Activated Protein C (APC). Activated protein C resistance is inherited as an autosomal trait with variable expression in heterozygotes. The homozygous state is not lethal, but has a higher rate of thrombosis 50- to 100-fold increase in risk than heterozygotes that have ~5- to 10-fold increase in risk of thrombosis. It is believed that the mutation is responsible for approximately 20 to 50% of cases of thrombosis in patients with presumed inherited thrombophilia.11 The most common clinical manifestations are venous thrombosis and pulmonary embolism, whereas the FV Leiden mutation is not a risk factor of arterial thrombosis.12 Prothrombin G20210A is the second most common inherited risk factor for thrombosis appears to be associated with arterial as well as venous thrombosis.12 Many patients with the prothrombin G20210A mutation have increased levels of prothrombin (about 30 percent above normal in heterozygotes and to 70 percent above normal in homozygotes) and increase thrombotic risk by at least two to three folds.13
A cross section study was conducted in Wad Medani Pediatrics Teaching Hospital in Wad Medani City, the capital of Gezira state. The Hospital represents the children reference hospital in Gezira state and neighboring statesThe study population consists sickle cell disease patients attending the hospital regularly and their relatives from both sex and all ages. The clinical state of the patients was determined by the physician in the haematology refer clinic in the hospital and considered as steady state when there is no need for admission to hospital. The patients were excluded if they were on medication such as anticoagulants, anti-aggregates or any interfering drugs.
A total of 50 patients diagnosed as sickle cell disease in steady state also 50 blood sample were obtained from healthy participant matched with age and sex excluding pregnant women and individuals with antecedents of thrombosis or taking any medication likely to interfere with the haemostatic system. Ethical approval was obtained from The General Administration of Planning and Research committee at Ministry of health, Gezira state, Sudan. Written or verbal consent were obtained before sample collection.
Coagulation screening
Platelets Poor Plasma (PPP) was prepared using citrated blood. PT, PTT and TT were performed on fresh plasma for both study subjects and controls. plasma for antigenic assay of D-dimer and TAT was kept in -20 until processing. Prothrombin Time (PT) was performed using STA: Neoplastic (plus /s) (REF 00606). INR is calculated by the following formula, with ISI (T/C) ISI Partial Thromboplastin Time (PTT) was performed using c.k.prest (2) catalogue No (ref 00598). Thrombin Time (TT) was estimated using STA thrombin2 (REF 00611). D-Dimer (D2D) was estimated using Human ELISA Kit, Catalog Number. CSB-E05175h from Cusabio–China. Thrombin-Antithrombin Complex (TAT) was estimated using Human ELISA Kit from abcam- Ab108907–USA.
DNA extraction
DNA was extracted by using commercial DNA extraction kit (Intron, Korea) according to the manufacturers’ instructions and stored at -20ºC until used.
Polymerase chain reaction (PCR)
PCR was performed and the test was carried out in a total volume of 20µl, PCR reaction containing 5 μl of the extracted DNA, 2 μl from the primers Factor V Leiden forward (5'CTTGAAGGAAATGCCCCATTA–'3) and reverse (5’TGCCCAGTGCTTAACAAGACCA-3’), Prothrombin Mutation 202021 Forward(5'-TCTAGAAACAGTTGCCTGGC-'3) Reverse (5-ATAGCACTGGGAGCATTGAAGC-3) and8μl of distilled water was added to the Dream Taq Green PCR Master Mix DNA marker “Gene Ruler” were provided by Thermo Scientific (Lithuania). The amplification for FV Leidnwas done by 35 cycles of PCR reaction (initial denaturation at 94oC for 5 minutes,denaturation at 94°C for 1 min, annealing at 56°C for 1min and extension at 72°C for 1 minute. A final extension was performed at 72°C for 5 minutes). Therestriction enzyme Mnl (5,000 units/ml) was used to digest a 267-bp amplified fragment, 10 μl of PCR product was digested with 5 unit of DNA restriction enzyme Mnl1 at 37oC for 30min and overnight, then product was separated on 4% agarose gel electrophoresis and the product was visualized by staining with 0.15% ethidium bromide using UV gel documentation system.The amplification for ProthrombinMutation202021was done by 40 cycles of PCR reaction (initial denaturation at 94oC for 5 minutes, denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C for 30 sec. A final extension was performed at 72°C for 5 minutes).product shows a segment of 345 bp.Therestriction enzyme Hind111(20,000 units/ml)was used to digest a 345-bp amplified fragment of patient, 10 µl of PCR product was digested with 10 unit of DNA restriction enzyme at 37oC Hind111 for 60min and overnight, theproduct was separated on 2% agarose gel electrophoresis and the product was visualized by staining with 0.15% ethidium bromide using UV gel documentation system.14
(Figures 1–8) & (Tables 1–3).

Figure 4 Means of coagulation tests in positive and negative prothrombin mutation in SCA (Higher level of PTT showed in positive patients).

Figure 5 Means of D-dimer/ng/ml in Positive and negative prothrombin mutation in SCA patients (Higher mean level in positive patients).
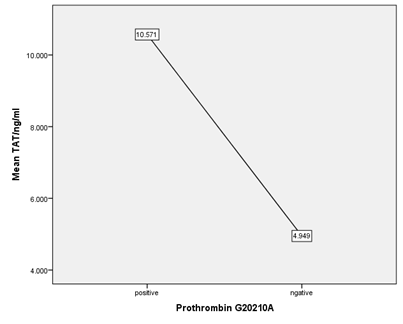
Figure 6 Means of TAT/ng/ml in Positive and negative prothrombin mutation in SCA patients (Higher mean level in positive patients).
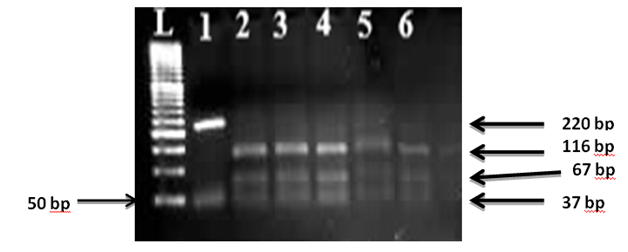
Figure 7 Wild type Fv (RFLP) L: 50 bp ladder, lane 1 Undigested 220 bp, lane 2-6 represents wild type fragments of 116,67 and 37 bp.
|
|
Clinical State |
Mean |
Std Deviation |
Sig (2-tailed) |
|
PT/sec |
Controls |
14.1326 |
2.70966 |
|
|
Patients |
16.5483 |
2.448 |
0 |
|
|
PTT/sec |
Controls |
32.4326 |
5.71159 |
|
|
Patients |
37.4122 |
6.54977 |
0 |
|
|
TT/sec |
Controls |
17.7609 |
2.30405 |
|
|
Patients |
19.2931 |
2.96339 |
0.004 |
|
|
INR |
Controls |
1.0465 |
0.18269 |
|
|
Patients |
1.1155 |
0.19711 |
0.068 |
|
|
D-Dimer/ng/ml |
Controls |
884.7 |
827.557 |
|
|
Patients |
2366.58 |
2849.188 |
0.001 |
|
|
TAT/ng/ml |
Controls |
2.39187 |
3.326668 |
|
|
|
Patients |
5.96136 |
6.716092 |
0.003 |
Table 1 Means of coagulation tests and P value in SCA patients and controls
|
|
Frequency in controls |
Frequency in SCA |
|
Prothrombin G20210A |
0% |
18.00% |
|
Fv Leiden |
0% |
0% |
Table 2 Frequency of positive inherited thrombophilia mutations in SCA patients and controls
|
|
Prothrombin G20210A |
Mean |
Std Deviation |
Sig |
|
PT/sec |
positive |
16.9556 |
1.98627 |
|
|
negative |
16.6049 |
2.65734 |
0.66 |
|
|
PTT/sec |
positive |
39.1667 |
4.27346 |
|
|
negative |
36.6832 |
7.05142 |
0.184 |
|
|
TT/sec |
positive |
19.3556 |
2.81874 |
|
|
negative |
19.4439 |
3.15508 |
0.935 |
|
|
INR |
positive |
1.1478 |
0.14575 |
|
|
negative |
1.1132 |
0.21332 |
0.565 |
|
|
D-Dimer/ng/ml |
positive |
3541.78 |
3332.065 |
|
|
negative |
2261.06 |
2731.699 |
0.312 |
|
|
TAT/ng/ml |
positive |
10.57133 |
10.91954 |
0.166 |
|
|
negative |
4.94941 |
5.057287 |
|
Table 3 Comparison of Means of coagulation tests between prothrombin positive and negative patients
Thrombotic phenomena have important roles in the vasocclusive manifestations in sickle cell disease (SCD), this study was conducted to identify the possible inherited risk factors which can associated with sickle cell gene and the possible association with activation of coagulation process. Prolong PT, PTT and TT and high INR were observed in study subjects comparing with control subjects (Figure 1) but with significant difference (Value .000, .000, .004 respectively which reflect activation of coagulation process occurs in SCA during steady state and exhibit high levels of thrombin generation markers and fibrinolytic system (TAT=5.96 ng/ml versus 2.39 ng/ml in controls with significant P.value.003,D-dimer level in SCD patients reach 2366 ng/ml versus 884ng/ml in controls with significant value 001 . This may be attributed to increase tissue factor expression even in non-crisis.15 It is suggested that free haemoglobin released upon haemolysis induce tissue factor expression of the blood vessels, and platelets activation.8 Other factors attributed to the activation of coagulation process, are exposure of erythrocyte phosphatidyl serin and erythrocyte ethanolamine which occurs due to elevation intracellular calcium (Ca2+) upon deoxygenation.8,16 As well as,enhanced circulating microparticles which originated from erythrocytes, white cells, platelets and endothelial cells as Monocyte Microparticles (MMP)which is considered as dominant source of Microparticles (MP)-borne TF and has been reported in sickle cell disease in many studies.17 Generally, vasocculssion events related to activation of coagulation pathway and impairment of the vessels.18
In our study FVL was completely absent in both controls and SCD patients as Only wildtype Fv (Figure 7) is dominate and this results approximately compatible with many previous studies which concluded that no significant associations between the SCA and Factor V G1691A (p=0.555).19,20 and other studies report similar prevalence of FV Leiden in both SCD patients and normal controls (21) suggesting that inherited hyper coagulability risk factors have low impact on the pathogenesis of sickle cell disease and its complications.
On the other hand, other studies reported higher percentage of FV Leiden mutation varied from 14% to 18.6% in SCD patients.21,22 A Meta-analysis showed that mutant genotypes (GA+AA vs. GG) of the FVL mutation was found to be higher in SCD patients with vasocculsion crises than in SCD patients without it revealed that FVL mutation as a high- risk factor for VOC in SCD patients).23
Our study showed positive heterozygous prothrombin G 20210 A Figure 8 in 18.0% of SCD patients versus complete absence in controls (0%), (Table 2) and this agree with a study showed a significant prevalence of heterozygous PRT G20210A mutation among patients (10.93%)21 and other study Heterozygous prothrombin G20210A mutation was (5.08%) in SS patients (allele A frequency 2.54%) and in controls (5.08%).24 Meta-analysis showed that the distribution of prothrombin G20210A mutation in SCD patients with or without vasocculsion is similar and revealed that the prothrombin G20210A is not associated with the risk of VOC in SCD patients14 Other studies reported completely absence of prothrombin mutation and no significant associations between the SCA and prothrombin G20210A mutation.19,22
In spite that the prevalence of the mutation SCD is variable in different populations but all studies consistent with evidence that it is a risk factor associated strongly with SCD but it’s clinical impact differ under many factors with evidence of a gene-gene and gene-environment interactions which enhance or inhibit gene expression in one side, on other side gene-nutrient interaction play a role in developing the related complications.
Comparison of coagulation tests PT.PTT and TT between positive and negative Prothrombin G2021A mutation within the SCD patients, positive patients showed slight prolongation in mean PTT (39.1667s versus 36.6832s in negative patient paralleled with slightly higher in mean level TAT (10.57133 versus 4.94941 ng/ml) and mean level D-dimer (3541. Versus 2261.06 ng/ml) suggesting that the prothrombin mutation may contribute partially in chronic activationcoagulation process in SCA patients even in non-crises situation in spite it is still statistically non-significant. (P value 0.184,0.312,0.166) respectively.
Activation of coagulation process occurs in SCD even during steady, heterozygous ProthrombinG20210 A present in 18% of the patients. FVL mutation was not reported in patients with Sickle Cell Anaemia.
Sanaa Hussein designed the research plan of this study and wrote the manuscript. Mohammed A. Mohammed carried laboratory analysis, and also contributed to the manuscript writing. Huda Haroon ,Babiker M. Ahmed, Elgaili M. Elgaili Adam Dawood coordinated the analysis of data, participated in the main role of editing the manuscript., Aisha Farhan Albadawi T. Abdelbagi, contributed to the manuscript writing and reviewing layout of results.final version of the manuscript was reviewed and approved the All authors.
None.
The authors declare there are no conflicts of interest.

©2020 Hussein, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.