International Journal of
eISSN: 2573-2889


Research Article Volume 5 Issue 3
1Ministry of Health, Kuwait
2Department of Physiology, Kuwait University, Kuwait
3Department of Anatomy, Kuwait University, Kuwait
Correspondence: Amr Osman, Department of Physiology,Faculty of Medicine, Kuwait University, Salmiya, Hawally, Kuwait, Tel 0096599986785
Received: August 21, 2020 | Published: September 30, 2020
Citation: Osman A, Bader MDA, Alqaryyan M, et al. Are BAX and JNK involved in inhibition of placental growth in rats with dexamethasone-induced intrauterine growth restriction. Int J Mol Biol Open Access. 2020;5(3):119-123. DOI: 10.15406/ijmboa.2020.05.00138
Molecular mechanisms involved in the onset and progression of intrauterine growth restriction (IUGR) are not clear, although enhanced apoptosis seems to play a primary role. The present study investigated two key proteins, B-cell lymphoma associated X protein and c-Jun N-terminal kinase, in the basal and labyrinth zones of Dexamethasone-induced IUGR placenta. Pregnant Sprague-Dawley rats received daily intra-peritoneal injections of either dexamethasone or saline starting from 14 days of gestation to 21. Dams were sacrificed on day 19 and 21; fetuses and placentas were collected. IUGR was seen at day 21 with significantly reduced fetal weights (p<0.05). Gene and protein expressions of B-cell lymphoma associated X protein and c-Jun N-terminal kinase in the placental basal and labyrinth zones were investigated by real-time polymerase chain reaction, western blotting, and immune histochemistry. Expression of both B-cell lymphoma associated X protein and c-Jun N-terminal kinase mRNA decreased in the basal zone and increased in the labyrinth zone (p<0.05) with no significant change detected at the protein level. The increase in expression of both B-cell lymphoma associated X protein and c-Jun N-terminal kinase in the labyrinth zone may indicate that both signaling molecules have a significant role in the apoptosis of this zone.
Keywords: BAX, JNK, placenta, dexamethasone, intrauterine growth restriction
ST, syncytiotrophoblast cells; BZ, basal zone; TG, trophoblast gaint cells; S, spongiotrophoblast cells; GC, glycogen cells; DC, deciduas cells; LZ, labyrinth zone
Rat and human placentas are known as hemochorial placentas sharing many similarities.1,2 Hemochorial placentas are considered to be the most invasive type of placentas, in which erosion of all maternal tissue layers allow for a direct connection between the maternal and fetal blood.3 Trophoblast cells are considered to be the parenchymal cells of the hemochorial placenta.2 In rats, the placenta is divided into two main zones, the basal zone (BZ) and labyrinth zone (LZ). The BZ consists of three types of differentiated cells: spongiotrophoblast cells (S), trophoblast giant cells (TG), and glycogen cells (GC).3 This zone is important in placental steroid production at the end of gestation.4 The LZ, responsible for nutrient and waste transfer between mother and fetus. It is made up of three layers of trophoblasts: outer trophectoderm and two layers of syncytiotrophoblasts (ST); they function as placental barriers.3
In rats, dexamethasone (DEX) has been used to induce intrauterine growth restriction (IUGR).5–7 IUGR is a condition in which the fetal weight is at or lower than the 10th percentile of the expected normal birth weight for the same gestational age or less than two standard deviations of the average weight of the fetuses.8 DEX is known to affect normal placental development by altering placental trophic function,9 altering trophoblast development10 and increasing trophoblast apoptosis.11 This method of treatment to induce IUGR provides a relevant model for placental experiments since glucocorticoids are used in the treatment of mothers at risk of preterm delivery12 and those who are affected by autoimmune disorders that need to be controlled by steroid therapy.
A healthy rat placenta normally experiences apoptosis that is time and zone dependent.7 In IUGR placentas, apoptosis rate is higher than in normal placentas towards the end of gestation.8 Apoptosis is an energy dependent process with two main pathways: intrinsic, also known as the mitochondrial pathway, and extrinsic, also known as the death receptor pathway.13 Both pathways culminate at an execution pathway mediated by caspase-3 and involve DNA fragmentation and eventual phagocytosis.13 The Bcl-2 family is a critical checkpoint of the intrinsic pathway.14 The Bcl-2 family includes pro-apoptotic and anti-apoptotic members, with B-cell lymphoma associated X protein (BAX) being an important pro-apoptotic member.15 BAX protein induces the release of cytochrome-C from mitochondria.16 The ratio of Bcl-2/BAX is important in setting the susceptibility threshold of the intrinsic pathway of apoptosis. BAX inserts into mitochondrial outer membrane in the form of homo-oligomerized multimers when a signal is received from caspase-8.14 In the mitochondrial outer membrane and liposomes, oligomerized BAX is able to form supramolecular openings, which is involved in the release of cytochorme-C from the mitochondria.17 Another Bcl-2 family member that is invovled in in the intrinsic pathway of apoptosis is Bcl-2 homologous antagonist killer (BAK). Following apoptotic activation signaling, BAK translocates from cytosol to mitochondria to release cytochrome C.18,19
C-Jun N-terminal protein kinases (JNK), which is also known as stress-activated protein kinase, is a protein that can affect gene transcription via phosphorylation of transcription factors.20,21 JNKs are a part of the mitogen activated protein kinases [MAPK] family that respond to different stress signals and play a role in the intrinsic and extrinsic pathways of apoptosis.22 JNKs induce apoptosis by transactivating certain transcription factors, therefore, upregulating the pro-apoptotic genes, or through acting on mitochondrial pro- and anti-apoptotic proteins by modulating their activity via specific phosphorylation events.23 Differences in JNK signal intensity and duration lead to different outcomes with short-term activation promoting survival and prolonged activation inducing apoptosis.24 JNK can mediate apoptosis directly through affecting the transcription of apoptotic genes and/or through a transcriptional-independent mechanism; also it can phosphorylate pro- and anti-apoptotic proteins and can prevent the ubiquitination and degradation of its substrates through phosphorylation. It is additionally involved in increasing the stability of p53 along with increasing its transcriptional activity and enhancing its apoptotic effect.21,25 JNK has a role in regulating BAX activity but it is not completely understood. It was shown that JNK can promote Bax translocation to mitochondria in stress-induced apoptosis through phosphorylation.26,27 This can be achieved through phosphorylation of 14-3-3, a cytoplasmic anchor of Bax, which lead to dissociation of BAX from the anchor and allowing translocation to the nucleus.27
In this experiment, DEX will be used to induce IUGR. We aim to explore the expression of BAX and JNK on the gene level by PCR and their translation into proteins through western blotting and localization within the cell using immune histochemistry.
Animal, experimental design, tissue preparation, and collection
Adult female Sprague-Dawley rats (280-300g) were kept in the Animal House at the Health Sciences Centre of Kuwait University and guidelines of the Animal House were followed. The rats were provided with food and water ad libitum and were kept on a 12:12 cycle of lightness and darkness in a room temperature between 20-22°C. For mating, adult male rats (280-300g) were placed with two females. The day of positive vaginal smear was considered day 0 of gestation. The pregnant rats were then weighed and kept in cages until day 14 of gestation. Pregnant rats were divided into two groups: a control group (C); which was given normal saline, and a DEX group; which received 0.4mg/kg DEX (Catalogue # D1159-500MG, Sigma-Aldrich Co., USA) as an intraperitoneal injection starting from the 14th day of gestation (dg) until the day of sacrifice (19 or 21 dg).
Laparotomy was carried out for the pregnant rats and uterine horns were obtained and placed on ice. Placentas were then acquired, cleaned and weighed, after which they were dissected for obtaining the basal zone (BZ) and labyrinth zone (LZ). Samples for PCR and western blotting were immersed in liquid nitrogen before storing at -70ºC. Whole placentas were placed in 10% formalin for immune histochemistry.
Real-time PCR
TRIzol (Invitrogen USA catalogue # 15596-018) was used for extracting total RNA as explained in our previous work.28,29 For each 0.1 g of sample, 1 ml of TRIzol was added and the samples were homogenized. After that, Chloroform (0.2 ml to each 1 ml TRIzol added) was added to each sample, after which the samples were centrifuged at 12,000 x g for 10 min. The aqueous phase was transferred to a new tube and ice-cold isopropanol was added and the mixture was incubated at room temperature for 10 min. The mixture then was centrifuged at 12,000 x g at 4°C for 10 min. The supernatant was removed, and 1 ml of 75% ethanol was added to the pellet. The samples were vortexed and centrifuged at 7500 x g at 4°C for 5 min. The supernatant was discarded and the pellets were left to air dry for 15 min. The pellets were then dissolved in 25μl of Diethyl pyrocarbonate (DEPC) water at 55°C in a thermo-mixer for 10 min.
Spectrophotometric measurements were used to determine the quantity of total RNA samples. The total RNA samples were diluted with DEPC water (1:500 dilution). The DEPC water was considered as blank and it was treated in the same way as the sample. Quartz cuvettes were used and the absorbance (A) was read at 260 and 280 nm. The purity of sample preparation was indicated by calculating the ratio of A260/A280; only samples with A260/A280 ratios (1.7-2.0) were used for further studies. RNA integrity was checked by electrophoresis using 1% (w/v) agarose gel and confirmed after visualization of the 28S and 18S rRNA bands under an ultraviolet (UV) transilluminator.
Before reverse transcription, the RNA samples (0.5μg/μl) were DNase-treated then reverse transcribed as described previously.30 The RT-PCR reactions were carried out for the following genes: BAX (#4331182 Rn 01480160_g1), JNK (Jkamp) (#4351372 Rn 01473305_m1) and the internal control 18S (catalog # 4319413E) using the TaqMan universal master mix (catalog # 4369016), all purchased from Applied Biosystems.
Western blotting and immune histochemistry
Placentas were thawed, homogenized and the protein was estimated. The samples containing 40μg of protein and rainbow marker (14,300 – 220,000 Daltons; catalog # RPN756E, GE Healthcare Ltd., UK) were loaded on gradient gels (SDS-PAGE 4-20%; catalog #0025244 Tris-HEPES-SDS, Precast Polyacrylamide Mini Gels). The run and protein transfer was performed as described previously.30 Proteins were transferred on PVDF membranes and then processed as follows; membranes were blocked with 10% non-fat dry milk in TBS-T (Tris-Buffer Saline-Tween 20: 20 mM Tris, 137 mM NaCl, pH 7.6 and 0.1% v/v Tween) for 1 hour at room temperature. Membranes were then rinsed and washed with TBS-T (20 ml/wash) and then incubated overnight at 4°C with 3 ml primary antibody diluted in 10% non-fat dry milk in TBS-T (BAX (P-19): sc-526; (1:200) (JNK (FL): sc-571 (1:200). After incubation, the membranes were rinsed and washed with TBS-T (20 ml/wash). The membranes were incubated with the appropriate secondary antibody diluted in 10% non-fat dry milk in TBS-T for 1.5 hours at room temperature. After washing, the membranes were covered with ECL plus (ECL Western Blotting Detection Reagents RPN 2106, GE Healthcare) according to the manufacturer’s instructions and exposed to films.30 After obtaining the results for BAX and JNK, the membranes were treated with β-actin antibody (internal control; Millipore Corporation, U.S.A. catalogue # MAB1501R)). Paraffin sections (5µm-thick) were deparaffinized using xylene and rehydrated in alcohol in declining concentrations of alcohol. The microwave oven was used for antigen retrieval using 0.01 M citrate buffer. For determining the expression of the required genes, relative quantification was used. Hydrogen peroxide was quenched by using 3% hydrogen peroxide and to minimize background staining a 1% sodium borohydride and glycine of the concentration of 50 mM were used. A blocking solution was then used as provided in the kit (cat # Ref 858943 Histostain ® plus Broad spectrum kit, Invitrogen, USA). The primary antibodies were diluted with PBS (primary antibodies were added to all slides except the –ve control to which PBS was added). The slides were kept in the humidified chamber for one hour at room temperature. Then, the slides were treated with the secondary antibody included in the kit (catalog # REF858943 Histostain ® plus Broad spectrum 1 kit, Invitrogen) for 30 minutes. Streptavidin was added (included in the kit; catalog # REF858943 Histostain ® plus Broad spectrum, Invitrogen) and kept for 30 minutes. The slides were rinsed with PBS twice, then ImmPACT-DAB chromogen-diaminobenzidine was added for two minutes then slides were visualized under the microscope, and color development was noted.
Dexamethasone induces IUGR and reduces placental growth in rats
Compared to respective control groups, DEX treatment resulted in reduced fetal body weight on 21 dg (p<0.05; Figure 1A). A significant reduction in total placental, basal zone, and labyrinth zone weights was seen on both 19 and 21 dg (p<0.05; Figures 1B,1C&1D). Based on these results, only 21 dg placental samples were used in this study to investigate the expression of BAX and JNK.
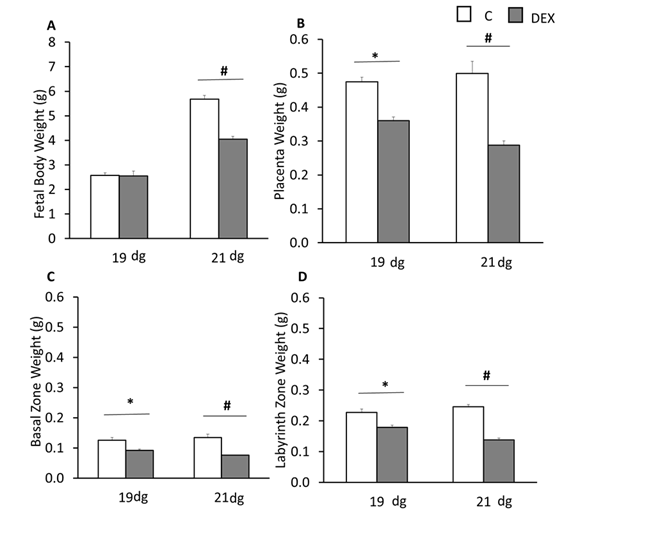
Figure 1 Effects of IUGR on fetal and placental weighs A, fetal body weight. B, placental weight. C, basal zone weight. D, labyrinth zone weight in control (C) and DEX (IUGR) groups at 19 and 21 days of gestation. *p<0.05 among C or DEX groups; #p<0.05 between C and DEX groups. Data represent mean ± S.E.M.
DEX affects BAX and JNK gene expressions in rat placentas
Both BAX and JNK mRNA expression decreased in the BZ in DEX placentas as compared to control placentas (p<0.05; Figures 2–3A). On the other hand, in the LZ, both genes increased in DEX placentas in contrast to control placentas (p<0.05; Figures 2A–3A).
DEX did not affect BAX and JNK protein expression in rat placentas
Both BAX and JNK were expressed in the BZ and LZ. A single band was detected for BAX protein (~21 kDa) while for JNK protein two bands were detected (~54 kDa and ~46 kDa). In both zones the expression of BAX and JNK proteins showed no significant change among the groups (Figures 2B&3B).
Localization of BAX in rat placenta
In the BZ, the glycogen cells (GC) and spongiotrophoblast cells (S) were stained with BAX in both control and DEX placentas. While in the LZ, syncytiotrophoblast cells (ST) were stained with BAX in both control and DEX placentas (Figures 2C–H).
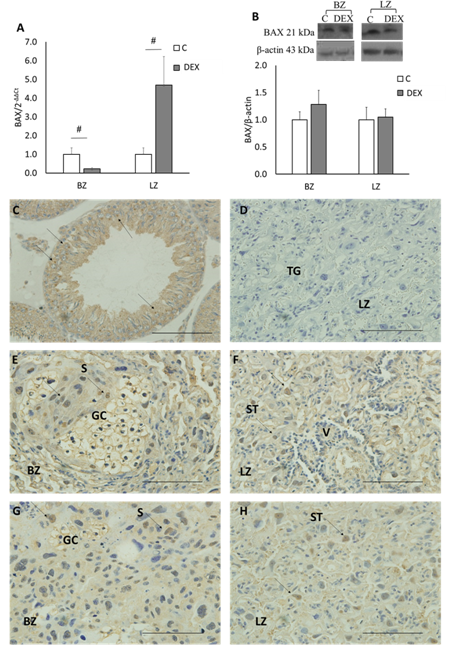
Figure 2 BAX gene, protein expression and localization in Basal (BZ) & Labyrinth (LZ) Zones. Bands of apparent molecular weight of ~21 kDa and ~43 kDa were detected for BAX and β-actin, respectively. A) BAX gene expression in BZ & LZ. B) BAX protein expression in BZ & LZ. #p<0.05 between C and DEX groups. Data represent mean ± S.E.M. C) The testis as a positive control for BAX using IHC staining. D) Negative control for BAX using IHC staining; cross section from rat placenta omitting the primary antibody. E & F) localization of BAX in C placenta sections. G & H) localization of BAX in DEX placenta sections. 40x magnification and a scale of 50 µm. ST: syncytiotrophoblast cells. BZ: basal zone. TG: trophoblast gaint cells. S: spongiotrophoblast cells. GC: glycogen cells. DC: deciduas cells; LZ: labyrinth zone.
Localization of JNK in rat placenta
In the BZ, trophoblast gaint cells (TG) were stained with JNK in both control and DEX placentas. While in the LZ, ST were stained with JNK in both control and DEX placentas (Figures 3C–H).
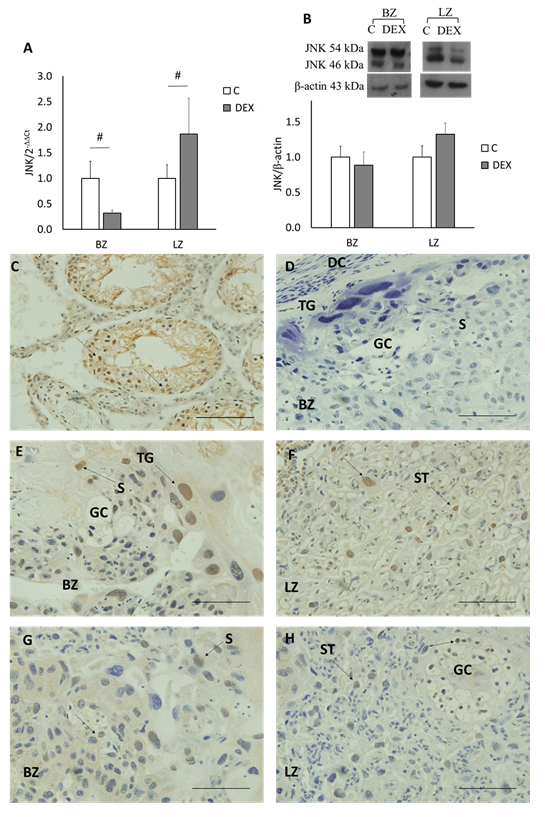
Figure 3 JNK gene, protein expression and localization in Basal (BZ) & Labyrinth (LZ) Zones. Bands of apparent molecular weight of ~54 & ~46 kDa (JNK) and ~43 kDa (actin) were detected. A) JNK gene expression in BZ & LZ. B) JNK protein expression in BZ & LZ. #p<0.05 between C and DEX groups. Data represent mean ± S.E.M. C) The testis as a positive control for JNK using IHC staining. D) Negative control for JNK using IHC staining; cross section from rat placenta omitting the primary antibody. E & F) localization of JNK in C placenta sections. G & H) localization of JNK in DEX placenta sections. 40x magnification and a scale of 50µm. ST, syncytiotrophoblast cells; BZ, basal zone; TG, trophoblast gaint cells; S, spongiotrophoblast cells; GC, glycogen cells; DC, deciduas cells; LZ, labyrinth zone.
Statistical analysis
All data are presented as mean ±SEM. Data were tested for statistical significance by analysis of variance (ANOVA) between control and experimental samples and between the groups 19 and 21 followed by least significant difference (LSD) post hoc analysis using SPSS software as described earlier30 (p<0.05) value was considered the lowest level of significance.
Since DEX is known to affect the normal placental development by increased levels of trophoblast apoptosis,11 it is therefore suggested that in DEX induced IUGR, apoptosis will be activated, leading to to a decrease in fetal body weight towards the end of gestation. In this study, JNK gene expression increased in the LZ suggesting an increase in cell apoptosis, as expected. This is also consistent with a previous research made by one of the members of our research, which showed up regulation of p53 expression in the LZ as JNK has been shown to stabilize and activate p53 being its substrate.30 The insignificant change in protein levels using western blotting maybe due to the presence of this protein in a single cell type in this zone. The decrease in the gene expression of JNK in basal zone indicates that other pro-apoptotic factors seem to play a role in decreasing the weight of this zone, suggesting that signaling molecules involved in apoptosis may be zone dependent.
Similarly, gene expression of BAX increased significantly in the LZ in DEX. This indicates that BAX contributes to apoptosis in the LZ, with the resultant decrease in placental weight in that zone. BAX gene expression in the BZ decreased, which may be due to that reduced BAX activation due to a change in phosphorylation by JNK27 which was shown to be decreased in the basal zone as mentioned above. The study done by our member using the same model showed that expression of p53 was increased in the LZ of DEX placentas, and it was observed that mobilization of p53 can induce apoptosis by up-regulation of pro-apoptotic factors such as BAX, reviewed by.31 This is consistent with our results which showed increased gene expression of BAX in the labyrinth zone. Apoptosis in the basal zone may be due to increased expression of other BCL2 family proteins, such as BAK,18,19 which can explain the decrease in weight in this zone. In our study, the level of BAX protein did not show significant difference between the control and experimental groups, this is most likely due to the analysis of all cell types in the LZ using western blotting, as previously mentioned. According to immune histochemistry results, only the ST cells stained positively for this protein.
In conclusion, our results indicate that BAX and JNK contribute to apoptosis in the labyrinth zone of IUGR placentas. The increased cell death in the BZ may be due to other pro-apoptotic factors of the BCL2 family members.
None.
The authors declare there are no conflicts of interest.

©2020 Osman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.