International Journal of
eISSN: 2573-2889


Research Article Volume 7 Issue 1
1Department of Genetics, Center for Biosciences, Federal University of Pernambuco, Brazil
2Center for Research in Biological Sciences - Nupeb, Federal University of Ouro Preto, Brazil
Correspondence: Maria Cidinaria Silva Alves, Department of Genetics, Center for Biosciences, Federal University of Pernambuco, Recife, PE, Brazil
Received: August 19, 2024 | Published: August 28, 2024
Citation: Alves MCS, Silva RCC, Quaresma S. Characterization of α-hairpinin in the genome of lima bean (Phaseolus lunatus). Int J Mol Biol Open Access. 2024;7(1):112-116. DOI: 10.15406/ijmboa.2024.07.00179
Antimicrobial α-Hairpinin peptides are sources of bioactive molecules with significant structural diversity and various biological activities. Phaseolus lunatus, known as lima bean, is a plant from the Fabaceae family, native to Central and South America. It is the second most important legume agronomically and economically in tropical regions, after the common bean. Due to its importance, several omics studies have been recently conducted on this species. The research aimed to characterize the antimicrobial peptides of the α-hairpinin family in the lima bean genome by analyzing their physicochemical properties, in silico biological activities, and three-dimensional structure. To this end, α-hairpinin sequences were identified according to the cysteine pattern characteristic of this protein family. After target identification, a search for conserved domains, signal peptides, and subcellular localization was conducted. Molecular mass, isoelectric point, grand average of hydropathicity (GRAVY), and biological activities were also analyzed, and a three-dimensional model of the candidate was generated. Following the mining process, an α-hairpinin sequence from P. lunatus (PlHrp1) was identified. PlHrp1 consisted of 41 amino acids, was predicted to be in the extracellular region, had an isoelectric point of 6.28, a mass of 4.81 kDa, and a gravy of -1.078. The alignment revealed that PlHrp1 contained the 4 conserved cysteine residues. In silico activity analyses demonstrated that PlHrp1 exhibited antifungal and antibacterial activities, with no antiviral activity. The secondary structure displayed two antiparallel α-helices connected by a loop. The model described 94.4% of amino acid residues in favorable positions. This study provides a comprehensive analysis of α-hairpinin peptides in the Phaseolus lunatus genome, highlighting the presence of this peptide family in the lima bean species and offering insights into their potential.
Keywords: bioinformatics, peptides, genomics, characterize, antimicrobials, α-hairpinin family
Cys, cysteine; aa, motif length; MW, molecular weight; pI, isoelectric point, GRAVY, average hydropathicity index; SVM, support vector machine; RFC, random forest classifier; Asp, aspartic acid; Lys, lysine; Arg, arginine
The family of α-hairpinin antimicrobial peptides was recently discovered in plants. Their sequences exhibit a characteristic signature motif (C1XXXC2-X(n)-C3XXXC4).1–3 The secondary structure of α-hairpinin proteins comprises two antiparallel α-helices connected by a loop (helix-loop-helix), stabilized by two disulfide bonds (Cys1-Cys4/Cys2-Cys3) in the tertiary structure.4,5 α-Hairpinin peptides are sources of bioactive molecules with significant structural diversity and various biological activities.2,6–8 They exhibit antifungal and antibacterial activities described in various species, such as barnyard grass (Echinochloa crus-galli),9 chickweed (Stellaria media),10 macadamia nut (Macadamia integrifolia),11 and wheat (Triticum kiharae).5 α-Hairpinins with trypsin inhibitory activity include BWI-2a and BWI-2b from buckwheat (Fagopyrum esculentum),4 C2 from pumpkin (Cucurbita maxima),12 and VhTI from ivy-leaved speedwell (Veronica hederifolia).6 Additionally, some α-hairpinins exhibit ribosome-inactivating activity, such as Luffin P1 from sponge gourd (Luffa cylindrica).13 Given these diverse biological activities, this family of short peptides, with potent antimicrobial properties and a simple structure, becomes a promising candidate for biotechnological approaches.1
Phaseolus lunatus, commonly known as lima bean or butter bean, is a plant belonging to the Fabaceae family, native to Central and South America. Lima bean is the second most agronomically and economically important legume species for humans in tropical regions, after the common bean.14 Due to the significance of this species, various omics studies have been conducted recently.15,16 This study aimed to characterize the antimicrobial peptides of the α-hairpinin family in the genome of the lima bean (Phaseolus lunatus), analyzing their physicochemical properties, biological activities in silico, and three-dimensional structure.
Genome retrieval
The genome of the lima bean was obtained from GenBank, hosted by the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/), with the accession code: 92715.16
Identification and characterization of sequences
The identification of α-hairpinin family sequences was performed using a Cysmotif Searcher17 with the cysteine pattern (C1XXXC2-X(n)-C3XXXC4), characteristic of this family.18,19 The candidate sequences were then submitted to InterProScan (https://www.ebi.ac.uk/interpro/search/sequence/) for the identification of conserved domains characteristic of the α-hairpinin class. Signal peptide presence was predicted using the SignalP 6.0 tool,20 while subcellular localization was predicted using the ProtComp 9.0 tool from Softberry Inc. (Mount Kisco, NY, USA). Subsequently, the sequences were evaluated for molecular weight (MW), isoelectric point (pI), and grand average of hydropathicity (GRAVY) using the ExPASy ProtParam software (http://web.expasy.org/protparam/). The conserved patterns of the sequences were analyzed through multiple alignment using Clustal Omega.21 The candidate sequences were evaluated for antimicrobial activity using the ClassAMP tool,22 antifungal activity using Antifp,23 and antiviral activity.24
Three-dimensional modeling
The sequences were evaluated using AlphaFold25 to select the homolog with the highest identity.19 The models were generated by MODELLER26 and selected based on structural evaluation through the DOPE-Score (Discrete Optimized Protein Energy) metric.27 To assess stereochemical conflicts in the models, PDBsum28 was used via the PROCHECK tool. PROSA-Web was employed to calculate the model folding (Z-score),29 and QMEANDisCo was utilized to evaluate the overall quality of the models.30 For analyzing biomolecular solvent-solute interactions, the APBS-PDB2PQR software (http://server.poisonboltzmann.org) was used, with the PROPKA algorithm applied for protonation at pH 7 and the CHARMM force field.31 The Poisson-Boltzmann equation was used to calculate solvation energy. The models were visualized using PyMOL (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC).
After the mining process, an α-hairpinin sequence from P. lunatus (PlHrp1) was identified. This motif was found in the protein with the Vicilin-like domain (Table 1), as observed in plant species such as cotton (Gossypium hirsutum).32
|
Gene |
Sequence |
|
PlHrp1 |
MKINLSLPILLFFLWALFSNLSLAKKETKVVGDPELKTCKHQCLQQQQYAEDDKLTCLR SCDVYHGLKHEREKQIEEKIREKEHEGSPEEHEEEEESSEKKEEEVEEGGEEEEEYPYIFEE DTDFDTKFETEDGRIRLLKKFTEKSNLLKGIENIRLAIVEAKAHTFVAPRHFDSDVVLFNIKG RALLGWVKESKTEKFILESGDMLAIPAGTQVYIVNRDENEKLFLAMLHVPVSTPGKFQEFF GPGGRDPESFLSAFGWNVLQAALQSPKRELEKLFNQQSRGSIFQISREKVQQMAPKKTSWW RFGGPSMAEFNLFTKPPTFSNRHGRLTQVGPPDSRSTLLEKLNTMLSFTNITKKSMSTIHYNS HATEIALVIDGKGHLQIVCPHISSRSNSKHEKSSPSNHRISAELKPGVLFVVPPGHPFVTFSSR EENLQIISFEINARDNKKFTFAGKDNIVSSMDDLAKELAFNYPSETVNKIFDRKESFFFPFELP RSDPRADA |
Table 1 Complete sequence with the α-hairpinin motif in red and the Vicilin-like domain highlighted in pink color
PlHrp1 consists of 41 amino acids, is predicted to be located in the extracellular region, and has a pI of 6.28, a mass of 4.81, and a GRAVY of -1.078 (Table 2). α-Hairpinins are antimicrobial peptides with low molecular mass (10 kDa), and to date, only a few α-hairpinins have been reported, including MBP-1, MiAMP2s, Ec-AMP1, Luffin P1, VhT1, BWI-2c, Tk-AMP-Xs, and Sm-AMP-X.1,2
|
Gene |
Gene-ID |
Length (aa) |
pI |
MW |
GRAVY |
Subcellular location |
|
PlHrp1 |
Pl07G0000309900.1 |
41 |
6.28 |
4.81 |
-1.078 |
Extracellular |
Table 2 Gene name, motif length (aa), molecular weight (MW), isoelectric point (pI), average hydropathicity index (GRAVY), and subcellular location of PlHrp1
The alignment results revealed that PlHrp1 exhibited the four conserved cysteine (Cys) residues, which formed two disulfide bridges with the characteristic pattern of α-hairpinin (Figure 1). α-Hairpinins are small, cysteine-rich proteins found in plants and fungi, known for their defensive functions. The structure of these proteins is distinctly characterized by a helix-loop-helix motif. This structural configuration is crucial because the four cysteines form two disulfide bridges, which are essential for the stability of the protein's three-dimensional structure.1,33 The formation of these disulfide bridges is a process that stabilizes the protein structure, helping to maintain its correct conformation and, consequently, its functionality. This stability is particularly important for α-hairpinins, which are often involved in interactions with pathogens or in responses to environmental stress.1,2,4,7,34–36 The structural stability conferred by the disulfide bridges is responsible for the high similarity in the spatial structure of α-hairpinins, meaning they maintain a similar three-dimensional shape despite differences in their amino acid sequences. This indicates that, although the amino acid sequence may vary significantly among different α-hairpinins, the overall helix-loop-helix structure and disulfide bridges are conserved.34 This high structural similarity, coupled with low sequence homology, suggests that α-hairpinins can evolve rapidly in response to different selective pressures while retaining the essential functionality provided by their three-dimensional structure.1,19
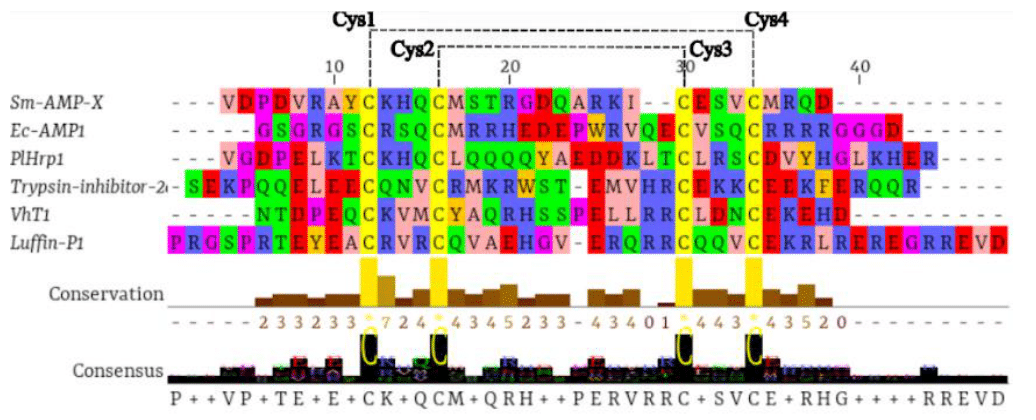
Figure 1 Multiple alignment of PlHrp1 with α-hairpinins from EcAMP1 of Echinochloa crus-galli (P86698); Luffin P1 of Luffa aegyptiaca (P56568); Sm-AMP-X (U4N938); Trypsin inhibitor 2c (P86794); VhTI of Veronica hederifolia (P85981). The dotted lines indicate the formation of two disulfide bridges.
This functional variety is reflected in the wide range of biological activities that α-hairpinins can perform. They may be involved in processes such as pathogen defense, cell signaling, and response to environmental stress, demonstrating significant adaptive plasticity.2,33,37 This makes α-hairpinins a topic of great interest in biotechnology and biochemistry studies, as understanding the basis of this functional diversity could open new avenues for the genetic manipulation of plants to enhance resistance to pathogens or adverse environmental conditions. The in silico antimicrobial activity analysis revealed that the PlHrp1 protein exhibits antifungal activity with a score of 0.93, indicating strong potential against fungi, and antibacterial activity with a score of 0.55, suggesting moderate efficacy against bacteria. However, PlHrp1 did not demonstrate significant antiviral activity, as shown in Table 3. This result is consistent with the observed profile of other α-hairpinins, which generally show specificity for fungi and bacteria, but not for viruses.
|
|
ClassAMP |
|
|
|
Meta-iAVP |
|
|
Gene |
SVM |
SVM |
RFC |
RFC |
Prediction |
Score |
|
PlHpr1 |
Antifungal |
0.935 |
Antibacterial |
0.552 |
Non-AVP |
0.176 |
Table 3 Antimicrobial and antiviral potential of PlHrp1. SVM, support vector machine; RFC, random forest classifier
The in vitro antimicrobial activity of various α-hairpinins has been well documented. For example, the α-hairpinin from maize (MBP-1) has been reported to be effective in resisting grain infections by pathogenic fungi and bacteria, such as Fusarium moniliforme and Fusarium graminearum, as well as demonstrating activity against Escherichia coli and Clavibacter michiganense.35 This observed in vitro antimicrobial effect for MBP-1 suggests that α-hairpinin plays a significant role in plant defense against a variety of pathogens. Additionally, the suppression of bacterial and filamentous fungal growth has also been observed in α-hairpinins from other species, such as Stellaria media,10 Triticum kiharae 5 and Echinochloa crus-galli.38 These studies indicate that the antimicrobial activity of α-hairpinins is not exclusive to a single species, but rather a common property among different plants and crops. The presence of antimicrobial activity in these α-hairpinins can be attributed to their ability to bind to and destabilize the cell membranes of pathogens, as well as the formation of disulfide bridges that may interfere with the vital functions of microorganisms.39–41 The lack of antiviral activity can be attributed to the specificity of α-hairpinins for different types of microorganisms, which is an aspect to consider when developing strategies for use in viral disease control.
Structural modeling was performed using the Phaseolus vulgaris protein (V7BFL3) as a template, which shares 90% identity with PlHrp1. This high degree of identity suggests that the modeled structure of PlHrp1 could be quite accurate, given the strong similarity between the template and the target.33 The modeled secondary structure of PlHrp1 revealed the presence of two antiparallel α-helices connected by a loop (helix-loop-helix) (Figure 2). This configuration is typical of α-hairpinins, a class of peptides known for their compact and highly conserved structure. The helix-loop-helix motif is a distinctive feature that imparts these proteins with a stable and functional structure.33 Despite the low sequence homology among different α-hairpinins, the three-dimensional structure and functional signature based on the Cys (cysteine) motif are preserved. This motif is crucial for the formation of disulfide bridges, which are essential for the protein's structural stability. The presence of two antiparallel α-helices connected by a loop contributes to the formation of a compact and structured core, which is protected by these disulfide bonds.1,2
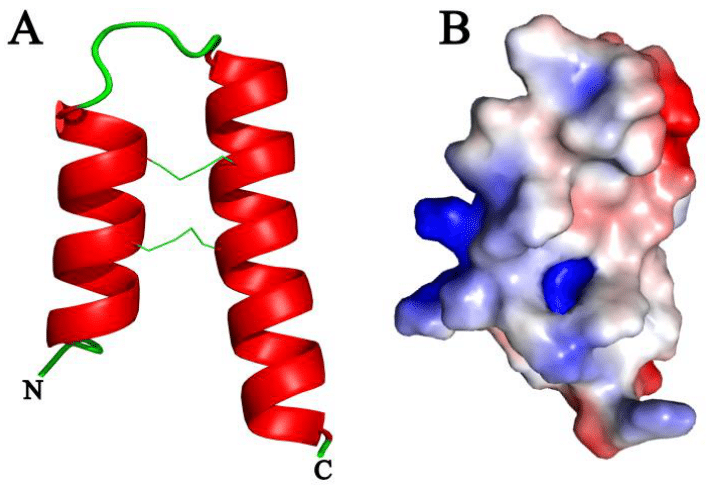
Figure 2 A, Three-dimensional model of PlHrp1, showing the disulfide bonds and the N-terminal and C-terminal regions; B, Surface electrostatic potential of the PlHrp1 model, with red indicating acidic charge, blue indicating basic charge, and white indicating neutral charge.
The structural signature based on disulfide bridges and the helix-loop-helix motif enables α-hairpinins to perform a wide range of biological functions, despite variations in their amino acid sequences. This specific three-dimensional configuration not only provides stability to the protein structure but may also be responsible for its ability to bind to specific targets, which is crucial for the observed antimicrobial activity.1,2 The low sequence homology among α-hairpinins may allow for rapid and efficient adaptation to different selective pressures, contributing to the functional diversity observed within this peptide family.3,42 Practically, this means that although different α-hairpinins may have distinct amino acid sequences, they can maintain a similar structural configuration that endows them with common functional properties. The characterization of the electrostatic potential map revealed that PlHrp1 exhibits regions with acidic, basic, and neutral surface charges (Figure 2). These charge variations are attributed to the specific amino acid composition in the protein's primary structure. Acidic and basic regions result from the presence of amino acid residues with charged side chains, such as aspartic acid (Asp) and lysine (Lys), respectively, while neutral regions are formed by amino acids with uncharged side chains.
For comparison, the MBP-1 peptide, another α-hairpinin, displays a predominantly positive surface charge. This is due to the high concentration of arginine (Arg) residues, which have strongly basic side chains. This characteristic is consistent with the findings of Sousa et al.43 who observed that the predominant presence of arginine imparts a positive character to the peptide. Differences in electrostatic potential among different α-hairpinins are expected and reflect the low sequence identity between peptides of this family. Although α-hairpinins share a similar three-dimensional structure, variations in their amino acid sequences can result in significant differences in electrostatic properties. These variations are a reflection of the functional and adaptive diversity of the family, as discussed by Tam et al.2 and Slavokhotova and Rogozhin.1 These differences in electrostatic potential may influence the interaction of α-hairpinins with their biological targets. Regions with specific charges can affect binding affinity and the specificity of interactions with pathogens or other target molecules. For instance, positively charged regions may bind to negatively charged cell membranes, while acidic or basic regions may interact differently with other proteins or molecules.
The stereochemical analysis of the model revealed that more than 94.4% of the amino acid residues were positioned in favorable conformations, as shown in Figure 3A. This high percentage of residues in favorable positions indicates a robust and stable structural conformation, reflecting the overall good quality of the three-dimensional model. Regarding the quality of the folding, the obtained Z-Score was -2.91, as illustrated in Figure 3B. The Z-Score is a measure that assesses the conformity of the model with known reference structures. A negative value indicates that the model is slightly outside the expected range for typical foldings, but still within an acceptable range. This value suggests that while the model is reasonably good, there may be some areas that are not as well-fitted as ideally expected. The estimation of the global energy function by the QmeanDisCo algorithm, which showed a global energy of 0.71, also provides valuable insights into the quality of the folding. The QmeanDisCo algorithm evaluates the overall stability of the structure based on various criteria, including the quality of atomic contacts and the general conformation. The presence of energetic peaks in the terminal regions of the model, observed in the N and C-terminal tails, indicates that these areas are less structured and therefore more flexible. This is consistent with the observation by Slavokhotova and Rogozhin,1 who highlighted that the ends of α-hairpinins often exhibit lower structural stability due to their lack of conformational definition.
This study provides an analysis of the α-hairpinin antimicrobial peptide family in the genome of Phaseolus lunatus. The identification of the PlHrp1 sequence, with its four conserved cysteine residues and adherence to the characteristic pattern of the family, highlights the presence of this family in the lima bean. In silico analyses revealed antimicrobial activities, and three-dimensional modeling offered valuable insights into the structure of PlHrp1. This research not only enhances our understanding of antimicrobial peptides in P. lunatus but also establishes a solid foundation for future investigations into the therapeutic and biotechnological potential of these compounds in agriculture and human health.
None.
The authors declare that there are no conflicts of interest.

©2024 Alves, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.