International Journal of
eISSN: 2573-2838


Short Communication Volume 4 Issue 6
1Department of Computer Science, City University of New York, USA
2 Department of Computer Engineering Technology, New York City College of Technology, USA
Correspondence: Sunghoon Jang, Department of Computer Engineering Technology, New York City College of Technology,186 Jay Street, 635 Voorhees Hall Brooklyn, NY 11201-2983, USA
Received: October 30, 2018 | Published: November 12, 2018
Citation: Zhang X, Jang S. The application of electric cell-substrate impedance sensing (ECIS) biosensors. Int J Biosen Bioelectron. 2018;4(6):26010.15406/ijbsbe.2018.04.00136
Electric cell-substrate impedance sensing (ECIS) is a label-free and non-invasive technique for analyzing the activities and morphologies of cells. The ECIS sensors are able to measure the impedance spectroscopy of cells attaching on the sensor substrates. The cell activities and morphologies influence the measure impedance directly. Traditional toxicity analysis has complicated processes compared to biosensor-based analysis. In this study, the toxicity analysis was performed with biosensor based on ECIS technique. The experimental results show that the ECIS sensor is able to quickly distinguish the toxic and non-toxic substance.
Keywords: ECIS, biosensor, toxicity analysis
ECIS is a popular technique, which is able to analyze cell behaviors by measuring impedance spectroscopy.1,2 The impedance spectroscopy provides information related to cell behavior and morphology, such as attachment, proliferation, barrier function, and migration.1–4 One ECIS sensor is usually composed with two or three electrodes, the working electrode and the counter electrode or reference electrode. The reference electrode could provide the standard reference electric potential for electrochemical measurement. During ECIS measurement, the live cells attach and spread on the surface of electrodes of ECIS sensors after seeding. The cells behave like an insulating layer and limits the current flow between the electrodes.5,6 As a result, the measured impedance increases right after the cell seeding. The measured impedance will be stable when the cells form a monolayer on the electrodes several hours after seeding. The impedance fluctuates slightly in such dynamic environments, where cell migrate, deform and attach/detach.7–11 Any variations caused by cell-cell interactions, cell-substrate interactions, or changing cell electrical properties due to chemical, biological, or physical stimuli,2,11 will influence the measured impedance spectroscopy. Besides the analysis of cell, ECIS can also be used in analyzing the toxic influence on live cells. Endothelial cells locating on the inner wall of vessels and contact the toxicants directly. Using endothelial cells in toxic testing by ECIS sensors can mimic the body’s response to toxicants. Cells lose their dielectric properties, when they are apoptotic after contacting toxicants. The cells monolayer tend to degrade or even detach from the sensing electrodes, which cause the measured impedance decrease dramatically.
Cell culture
Bovine aortic endothelial cells (BAEC) are used in this study (VEC Technologies, Rensselaer, NY). The BAECs were cultured in cell culture medium. The medium is Minimum Essential Medium (MEM, GIBCO, Grand Island, NY) with 10% fetal bovine serum (FBS, GIBCO, Grand Island NY). The cells are cultured under standard culturing conditions (37°C and 5% CO2).
Experimental system setup
The cell impedance was measured with impedance analyzer Agilent 4294 and ECIS Z system (Applied Biophysics, Troy, NY). The BAECs are seeded on the ECIS sensors before impedance measurements. All the ECIS sensors are kept in the incubator, which provide standard cell culture environment. The experimental setup is shown in (Figure 1) (Figure 2) shows the cell morphology right after seeding.
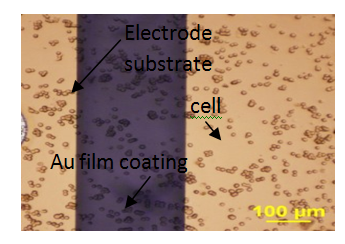
Figure 2 Show the BAEC attach on the multi-electrode arrays surface, 0.5 hour after seeding onto ECIS electrode arrays, seeding density are 150 K/cm2, 125 K/cm2 and 100 K/cm2 respectively.
Toxicity testing
In the toxicity testing, the ECIS sensor (Applied Biophysics, Troy, NY) was used to investigate the influence of lead oxide (PbO) on BAEC cell. Prior to the cell inoculation, suitable amount of 30µg/ml fibronectin (GIBCO, Grand Island NY) was coated on the surface of sensor to improve cell attachment. The fibronectin coating is kept on the sensor surface for 30 minutes in cell culture incubator before cell seeding. The cell suspension of BAECs is prepared in advance. BAECs were seeded into each culture to satisfy the final cell seeding density of 125,000cells/cm2. The PbO powder was mixtured with Dulbecco’s Phosphate Buffered Saline (DPBS, Media tech, Inc. Manassan, VA) to obtain 0.01mM PbO suspension as the toxicant in this study. During the toxicity testing, cell culture medium is replaced by PbO suspension for every ECIS sensor except the control after the cell form a monolayer, which is confirmed under microscope. The cell impedance was measured and analyzed to evaluate the toxic influence. Figure 3 shows the normalized impedance response by introducing 0.01mM PbO. The normalized impedance was calculated by dividing the measured impedance by the impedance right before introducing toxicant. The normalized impedance values decrease to 0.62 times its original value within 5 hours after introducing toxicant. For the control, the normalized impedance does not change dramatically compared to non-control samples. The impedance decrease indicates the degradation of cell layer due to the toxic effect of PbO. The cells change the morphology or even detach from the sensor surface. The intercellular junction also become weaker than that before toxicant introduction. The cell layer is considered to be an insulation layer between the electrodes of ECIS sensor. The cell detachment or weak intercellular junction will allow the ions move between the electrodes freely. As a result, the measured impedance decreases gradually. Therefore, ECIS can be used to indicate the toxicant effects.12,13
In this study, the ECIS sensors were used to perform toxicity analysis of PbO on BAECs. The impedance response could clearly identify the toxic effect of PbO.
None.
Authors declare that there is no conflict of interest.

©2018 Zhang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.