International Journal of
eISSN: 2573-2838


Research Article Volume 5 Issue 5
1National University Heredia, Costa Rica
2National Nanotechnology Laboratory, Costa Rica
Correspondence: Jose Vega-Baudrit, Chemistry School, National University Heredia, Costa Rica
Received: September 15, 2019 | Published: October 22, 2019
Citation: Gamboa SM, Rojas ER, Martínez VV, et al. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int J Biosen Bioelectron. 2019;5(5):166-173. DOI: 10.15406/ijbsbe.2019.05.00172
A great interest for the study of nanoscale chemical species has been studied. This review presents the main methods of chemical reduction for the preparation of silver nanoparticles, such as the preparation of silver particles using NaBH4 and ascorbic acid as a reducing and stabilizing agent, the preparation of silver particles using PVP as a reducing agent and the preparation of silver particles using DMF as a reducing agent. In addition, the main methods of characterization of silver nanoparticles are presented according to the size and morphology of the nanoparticles and the properties of surface and stability. Finally, the applicability of silver nanoparticles as an antibacterial agent is demonstrated.
Keywords: silver nanoparticles, chemical reduction method, particle size, AFM, DLS, SEM, TEM, EDS, FTIR, antibacterial agent
EDS, energy dispersive X-ray spectroscopy; FT-IR, infrared spectroscopy or Fourier transform; DLS, dynamic light scattering; PCS, photon correlation spectroscopy
At present a great interest has been visualized by the study of chemical species of nanometric size (Figure 1), this due to the applicability that has been demonstrated in chemical research areas due to its great variety of properties. Their scale of size and their metallic character makes them even more interesting in their practical application due to their biological, optical, catalytic properties, etc.1 In the nanoparticles of metals, the optical properties focus on the mass oscillation of the free conduction electrons as a result of the interaction with the electromagnetic radiation, the electric field that is formed induces the formation of a dipole in the nanoparticle which is what attributes its restorative force due to the attempt to compensate for this effect, developing in parallel the study of properties and their multiple applications.2 Silver has many uses, but undoubtedly one of the most interesting is its use as a disinfectant agent for antibacterial purposes. Due to the great boom that has existed in nanotechnology, different physical, chemical and biological methods have been developed for obtaining silver nanoparticles, so this paper seeks to describe some of the methods, their characterization and paying special attention to their capacity as an antibacterial agent.3

Figure 1 Photos of silver nanoparticles in the image nanowires, nanocubes, nanopyramides, nanoprisms.4
Chemical synthesis of silver nanoparticles
The nanoparticles have been of great scientific value since they came to reduce the gap between the bulk materials and the atomic / molecular structures, the nanoparticles of silver are the most attractive due to the high surface area by volume ratio, the surface of the Nano particles are so important and should be controlled since a change in the size of the surface can generate a change in the physical and chemical properties of the nanoparticles.4 But what does the properties of these nano particles depend on? What can we control so that the properties of them vary? When the particles reach a size between 1-100nm their properties are different at the electrical, chemical and physical levels, so it is evident that the properties are directly related to the size, so that by changing their size and shape, control is achieved of properties such as: temperature, redox potential, its color, conductivity, chemical stability, electrical qualities, optics, etc.5 Extensive studies have shown that the size, morphology, stability and properties specifically of the nano silver particles are greatly influenced by the experimental conditions of their synthesis, the kinetics of the reaction, the interaction of the ions with the reducing agents and the absorption processes of the stabilizing agent used.5 so that the specific control regarding its shape, size, distribution of the desired silver nano particle falls on the synthesis method that is selected.6 Most chemical syntheses are based on the reduction reactions of metallic silver salts, but first you must select the shape of the nano particle that is sought, if it is spherical, if it is triangular, cubic, pyramidal, rods, cylinders, (Figure 2) once the form is known, then the method that best fits the nanoparticle shape is selected, since the speed of the reaction and the interaction with the stabilizers define the shape of the nano particle (Figure 3). It should be known, what happens in a nanoparticle synthesis process? As mentioned above requires a precise control in the synthesis to control the size and shape in order to obtain a set of particles with a certain property. In a synthesis by the general we have the following components which must be known to manipulate and work them: metallic precursor, reducing agent (solvent) and stabilizing agent (Figure 4). In addition, two very important formation processes are taken into account, one is nucleation, in which a high activation energy is required for the agglomeration of the atoms and the other is that of growth where, on the contrary, a low energy of Activation for ordering in the formation of particles, is in these points of the synthesis where the shape and size are totally dependent on the speed of both processes which are controlled by parameters such as concentration, temperature, reducing power and the pH.7 On the other hand, the stabilizing agent plays a very important role in the synthesis because with its help the nanoparticles are protected in such a way that an unexpected agglomeration is prevented in the step of controlling their size and shape (Figure 5).
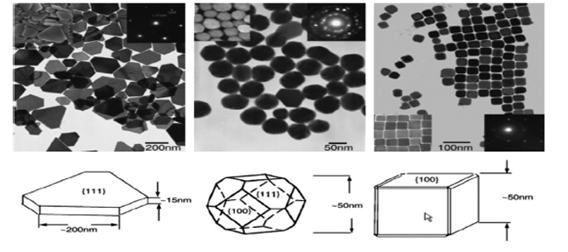
Figure 2 TEM images of silver NP (triangular (left), hemispherical (medium) and cubic (right) Ag nanoplates compatible in Cu-TEM grids and their structural models Study of styrene oxidation in three forms of nanoparticles silver.4
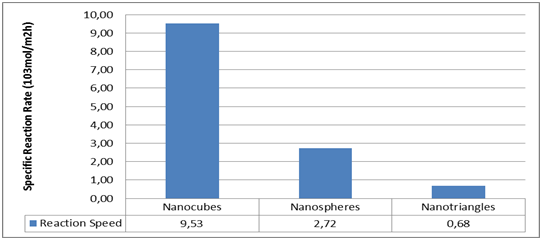
Figure 3 Study of the oxidation of styrene in three forms of nanoparticles (triangular, nano spherical and cubic plates) of silver The results of the graph show that the speed of the cubic nanoparticles is 14 times more than the triangular ones and 4 times more than the hemispherical ones.4
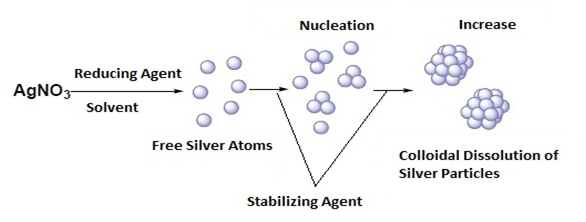
Figure 4 General mechanism of formation of silver nanoparticles from the chemical reduction in solution of AgNO3 salt.8
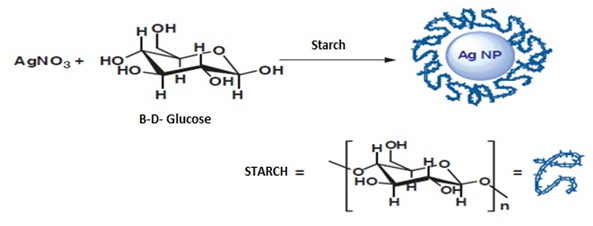
Figure 5 Silver nanoparticle synthesis method where starch functions as a stabilizing agent8
There is a wide range of methods for the synthesis of silver nanoparticles (Table 1), where the participants we select in the reaction depend, so will the shape and characteristics of the Nanoparticles, then we will detail some of the chemical synthesis methods existing, where in its vast majority from Silver Nitrate are obtained Silver Acetate and Silver Citrate, which are recrystallized and purified to be used as precursors of silver nanoparticles. Different studies show that when silver citrate is used as a silver precursor, the solution was transparent and stable over time (for at least 3 months). While when using silver nitrate or silver acetate the resulting colloidal solution was unstable and silver precipitated after 1 day.5
Method |
Reducing Agent or Solvent |
Stabilizer or surfactant |
Particle size |
Shape |
Chemical |
Trisodium citrate |
Trisodium citrate |
30-6Onm |
Spherical |
Chemical |
NaBH4 |
Citrato |
7nm |
Spherical |
Chemical |
Ethyleneglycol |
PVP |
17±2nm |
Spherical |
Chemical |
Paraffin |
Oleylamine |
10- l4nm |
Spherical |
Chemical |
B-D-giucuose |
Starch |
|
Different |
Chemical |
DMF |
APS/PVP |
|
Different |
Chemical |
Hydrazine hydrate |
AOl |
2-5 nm |
Spherical |
Chemical |
Ethyleneglycol |
PVP |
30-50nm |
Cubic |
Chemical |
Pentanediol |
PVP |
|
Cubic |
Chemical |
Sodium Tartaric |
PVP |
|
Bars |
Chemical |
Ethyleneglycol |
|
30-40nm |
Wires |
Chemical |
Ethylene glycol |
PVP |
|
Bars |
Chemical |
UV Radiation |
PVP/PEG |
|
Sphere/Prism |
Chemical |
Hydrazine hydrate |
PVP |
50- 200nm |
Triangular |
Wet- Chemical |
Sodium borohydride/sodium citrate |
|
4 ± 2 nm |
Bars |
Chemical Solution |
Ascorbicacid |
CTAB |
|
Wires |
Wet-Chemical |
Ascorbicacid |
|
30-40nm |
Wires |
Physical |
Electrical arc discharge |
Sodium Citrate |
14-27 nm |
Spherical |
Physical |
D(-100, UV |
D(- 100 |
3Onm |
Spherical |
Green |
Extractos Vegetales |
Vegetable extracts |
30-40 nm |
Spherical |
Biological |
Bacillus sp |
Bacillus sp. |
5-15 nm |
Spherical |
Biological |
Lactobacilius |
Lactobacilius Proteins |
6- 15.7nm |
Spherical |
Biological |
Shewaneila oneidensis |
Shewaneila oneidensis |
2-11 nm |
Spherical |
Biological |
Fungus T. viride |
Trichoderma viride |
5-40 nm |
Spherical |
Biological |
Cassia angustifoila |
Cassia angustifoila |
9-31 nm |
Spherical |
Biological |
Daucus Carota |
Daucus Carota |
2Onm |
Spherical |
Biological |
Bacillus Strain CS 11 |
Bacillus Strain CS 12 |
42-92 nm |
Spherical |
Biological |
Aspergililus niger |
AspergiHius niger |
1-20 nm |
Spherical |
Biological |
Arbutus unedo leaf extract |
Arbutus unedo leaf extract |
3-20 nm |
Spherical |
Biological |
Leaf extracts from Eucalyptus macrocarpa |
Eucalyptus macrocarpa |
38 ± 2 nm |
Cubic |
Photochemical |
Carboxymethylated chitosan (CMCTS) |
CMCTS |
2-8 nm |
Cubic |
Microwavelechnique |
Ethyleneglycol |
PVP |
|
Wires |
Microwave-Assisted |
Ethylene glycol monoalkyl ethers |
PVP |
|
Prism |
Photo chemical reduction (X-ray radiolysis) |
X-ray |
|
28 nm |
Spherical |
Electrochemical (Poiloiprocess) |
Electrolysis cathode: titanium anode: Pt |
PVP |
11 nm |
Spherical |
Table 1 Synthesis of silver nanoparticles using different synthesis methods to obtain nanoparticles with different characteristics and sizes
Synthesis method 1: preparation of silver particles using NaBH4 and Ac. ascorbic as a reducing and stabilizing agent
From Silver Nitrate used as a precursor of silver nanoparticles, using ascorbic acid (99%), sodium borohydride as reducing agents also working silver nanoparticles with a concentration between 250-500mg/dm3 by the addition of silver as a precursor (silver citrate, silver nitrate or silver acetate) drop wise to an aqueous solution of sodium borohydride (Figure 6) silver nanoparticles were obtained by injecting NaBH4 solution into an aqueous solution of AgNO3 in the presence of citrate (Figure 7). In addition, a subsequent treatment to these nanospheres, using AgNO3, but now with ascorbic acid as a reducing agent, nanoparticles are obtained in the form of a rod / cable.5 The formation of these can be confirmed by means of a silver spectrum for the colloids that used silver citrate since a strong plasmon band is observed near 396nm, which confirms that the silver ions were reduced to Ag° in phase watery (Figure 8).
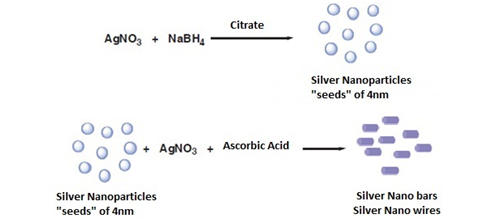
Figure 6 Method of chemical synthesis of nanospheres and silver nanobars.8
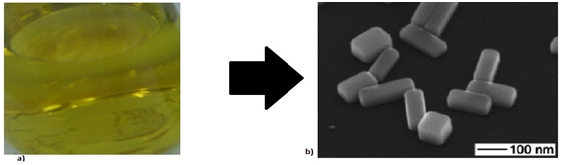
Figure 7 A. Preparation of a silver colloid in aqueous solution using sodium borohydride as a reducing agent.5 B. TEM image of silver nanobars.4
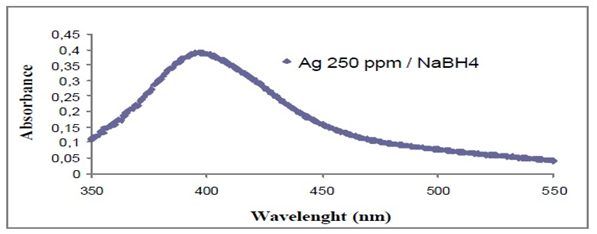
Figure 8 UV-VIS spectrum for a yellow silver colloid.5
Synthesis method 2: preparation of silver particles using PVP as a reducing
By means of this method of synthesis of silver, particles with various forms are obtained, simply other factors must be controlled (Figure 9). Using an aqueous solution and PVP as a stabilizing agent, the silver precursor is added, for example, to about 150mL of deionized water containing 5g of PVP for the formation of the silver colloid in a concentration of 250-500mg/dm3, adding drop wise to the silver nitrate or silver citrate solution and stirring for 1h.5 The poly (vinyl pyrrolidone) polymer (PVP) is one of the most widely used stabilizing agents for metallic nanoparticles. Thus, one of the first syntheses consisted in the photo reduction of AgNO3 in the presence of PVP as a stabilizing agent using 243nm UV radiation. With this method, silver nanoparticles between 15 and 22nm can be obtained depending on the molar ratio between AgNO3 and PVP. Subsequently, different methods have been described in which PVP acts as a stabilizing agent for silver nanoparticles synthesized by reducing silver salts with different chemical reducing agents such as potassium bitartrate or DMF and even using microwaves and the PVP itself as a reducing agent. PVP has also been used as a stabilizing agent in the reaction of AgNO3 with polyols (Polyol method) that leads to the synthesis of nanospheres, nanocubes, nano bars or silver nanowires. In general, the study of the parameters that affect the reactions and, especially, the concentration of the stabilizing polymer, allow exercising a great control over the size and shape of the silver nanoparticles.8
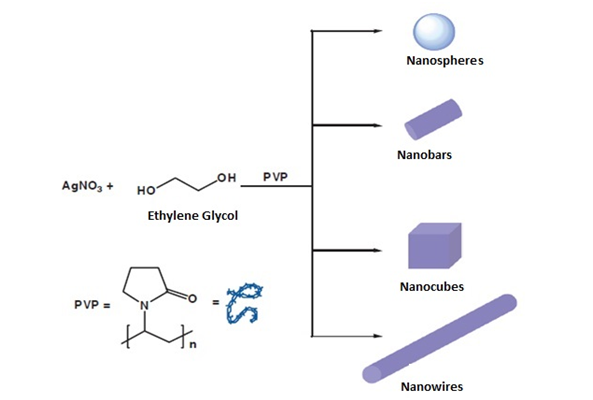
Figure 9 Different silver nanostructures synthesized by the reduction of silver nitrate in ethylene glycol, using polyvinylpyrrolidone as a stabilizing agent.8
Synthesis method 3: preparation of silver particles using DMF as a reducing
In this case, dimethylformamide (DMF) has been used as a solvent and as a reducing agent against silver salts under different reaction conditions (Figure 10). Thus, nanoparticles of different sizes have been achieved using aminopropyltriethoxysilane (APS) or polyvinylpyrrolidone (PVP) as stabilizing agents. When the PVP polymer is used as the stabilizing agent, silver nanoparticles (spherical or prisms) can be obtained. When APS is used, silver nanoparticles surrounded by a layer of silica are obtained. As mentioned above, the aggregation of the nanoparticles in solution can be prevented by the use of stabilizing agents. The process of forming the nanoparticles is usually similar to that described above, that is reduction of a silver salt in the presence of a reducing agent. However, the use of these agents allows, on the one hand, to avoid the aggregation of nanoparticles in organic solvents and, on the other hand, to exert precise control over their size, shape and monodispersity by modifying the reaction conditions.
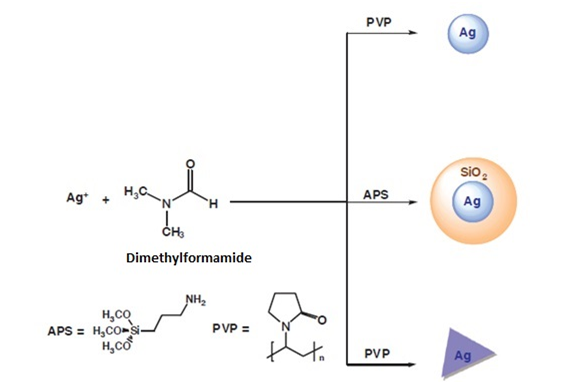
Figure 10 Synthesis of silver nanoparticles using DMF as a reducing agent.8
Synthesis method 4: green method, preparation of silver particles using natural precursors
Green sources include a wide range of natural precursors that include plant extracts, bacteria, and enzymes. One of the most common sources of antioxidants used for the synthesis of silver nanoparticles is fruit juices, as they can act as coating agents and reducers. One of the examples is the pomegranate that as a rich source of polyphenols has been the target of many studies for the determination of its constituents and the attribution of its properties to its available components in the shell, mesocarp, aril and juice. New biosynthetic sources were used to obtain Ag nanoparticles, in the solution phase, the pomegranate shell extract forms a complex of natural silver, which at high temperature (300°C) is transformed into silver nanoparticles (Figure 11). However, the same complex ends in bulk metallic silver at 600°C resulting in well dispersed silver nanoparticles. In addition, the solid state reaction of a natural precursor with the silver salt exposed to high temperatures results in fascinating and well dispersed spherical nanoparticles with homogeneous size and shape demonstrating the successful synthesis of silver nanoparticles with excellent morphological properties by a very easy, profitable and green method.7 The size and shape of the particles can be confirmed by characterization methods (Figure 12) for example after 70h of irradiation of silver particles, a decrease in the intensity of the characteristic surface of the plasmon band is observed in the spectroscopy UV-VIS for spherical particles. The spectra of the silver colloids contain a strong plasmon band near 410nm, which confirms that the silver ions were reduced to Ag° in the aqueous phase. It is found that as the silver concentration increased, the absorption band became sharper, the maximum absorption resulted in a red shift from 402nm to 407nm when the concentration of AgNO3 was increased from 250 to 1000mg/dm3 which mean that the particle size increased and large aggregates of silver were formed. Transmission electron microscopy (TEM) confirms that spherical silver particles became prismatic structures.5

Figure 11 Green synthesis of silver nanoparticles using pomegranate extract as a precursor.7
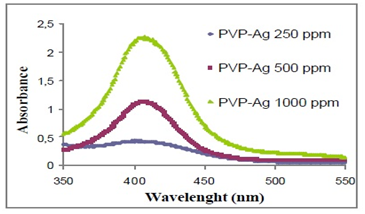
Figure 12 Spectrum of UV-Vis absorption of silver colloids containing different amounts of silver.5
Characterization of silver nanoparticles
The expansion of nanotechnology in various research areas has led to the need to use analytical techniques for the analysis and characterization of nanoparticles. Several characterization techniques are available, including microscopic, separation and spectroscopic techniques.9 Nanoparticles are usually characterized in the literature by their size distribution, morphology, surface properties, stability and interactions. The main characterization techniques of nanoparticles in general and AgNP in particular, are shown in Table 2.
Techniques |
Physicochemical characteristics analyzed |
X-ray photoelectron spectroscopy (XPS) |
Elemental and chemical composition at the surface |
Zeta potential |
Stability referring to surface charge |
Infrared spectroscopy (MS) |
Structure and conformation of bioconjugates, Functional group analysis. |
Scanning electron microscopy (SEM) |
Size and size distribution, Shape, Aggregation. |
Transmission electron microscopy (TEM) |
Size and size distribution, shape heterogeneity, aggregation. |
Dynamic light scattering (DLS) |
Hydrodynamic size distribution |
Near-field scanning optical microscopy (NSOM) |
Size and shape of nanomaterials |
Nuclear magnetic resonance (NMR) |
Structure, composition, purity. |
Mass spectrometry (MS) |
Molecular weight, composition, structure. |
Atomic force microscopy (AFM) |
Size and size distribution, shape, structure, Aggregation. |
Table 2 Principal techniques for evaluation of the physicochemical characteristics of nanoparticles
Size and morphology of the nanoparticles. (SEM, AFM, DLS)
Scanning electron microscopy is a surface imaging technique in which an electron beam interacts with a sample generating different signals, which reflect the atomic composition and morphology of the surface. SEM, uses backscattering electrons and secondary electrons emitted by the sample to construct the three-dimensional image of the sample analyzed. Once these electrons escape from the surface of the sample, they are detected by a photomultiplier. However, many nanoparticles are invisible to the electron microscope, because they do not deviate the electron beam enough, therefore, the preparation of the sample it requires a coating with a thin layer of metal that creates a conductive layer on the sample. This procedure inhibits surface wear, reduces thermal damage and improves the secondary electron signal required in the SEM. The size, size distribution and morphology of the nanoparticles can be obtained directly from SEM, the purity of the sample and its degree of aggregation can also be inferred from SEM images. However, this technique has the disadvantage that the preparation of the sample is destructive, and one cannot be sure that the observed image is truly representative of the sample, which could lead to biased statistics of the size distribution in samples heterogeneous. Atomic force microscopy (AFM) is an essential tool to study both surface morphology with nanometric resolution and for measurements of sensory forces. AFM images are obtained by detecting the attractive / repulsive forces between the sample surface and a sharp probe. The force is measured through a laser photodiode system that detects the difference in voltages at the photodetector output. Similar to the SEM, the AFM serves to study the size, distribution of size, shape and aggregation of the nanoparticles, with the advantage that the procedure of sample preparation is not destructive, besides that it is possible to obtain images of a large variety of biomaterials in aqueous fluids and observe the macromolecular movement of the sample in real time. AFM, has lower economic cost than SEM, besides requiring less space in a laboratory and it is much easier to operate, by using these two techniques in a complementary way, one will compensate the other. Another microscopy technique useful for the characterization of the shape and size is the transmission electron microscopy (TEM), the image is two-dimensional and is formed from two electrons transmitted through the sample, and this technique is very valuable to measure the polymer wall of the nanoparticles. Dynamic light scattering (DLS) is widely used in the determination of nanoparticle size, also known as photon correlation spectroscopy (PCS). In the operation of the DLS, a monochromatic light laser, which is dispersed in a photon detector, by the Brownian movement of the particles, illuminates a colloidal suspension, the intensity of the detected light fluctuates in time, and this is related with the size of the particles according to a correlation function. One of the main advantages of the DLS is that it provides information about the population of particles in the short duration of the experiment; DLS assumes that all particles are spherical in nature. Care should be taken when interpreting the size of the particles with this technique, because large particles scatter more light than small particles, but small amounts of aggregates or dust particles could cause larger values to be obtained. A great advantage of this technique is the ability to solve populations of multiple sizes with more precision. Generally, the DLS results are complemented by the images provided by AFM and SEM.
Surface properties and stability
The most common techniques for the analysis of surface properties are zeta potential, energy dispersive spectroscopy (EDS), photoelelectronic X-ray spectroscopy (XPS), infrared Fourier transform spectroscopy (FTIR) and Raman. These techniques are indicative of the chemical composition of the surface of the nanoparticles, the details of shape and size is only possible with microscopic techniques. Zeta potential analysis, used to determine the surface charge and stability of the colloidal suspension of nanoparticles, is important in determining the interactions of cell membranes. The analysis is carried out through the electrophoretic mobility of charged particles under an applied electrical potential. Energy dispersive X-ray spectroscopy (EDS) is a chemical analysis technique used in conjunction with electronic microscopy instruments such as SEM. It is based on the "fingerprint" energies of the X-rays emitted by the samples during the bombardment of an electron beam. It is also possible to perform a general mapping of the sample by executing the X-ray spectra in scan mode. Another technique that uses the excitation of X-rays to quantify the chemical composition is X-ray photoelectron spectroscopy (XPS). Infrared spectroscopy or Fourier transform (FT-IR) and Raman spectroscopy are two types of vibratory spectroscopy, useful for investigating the structural properties of nanoparticles. They are based on changes of dipolar moments (FT-IR) or polarizable (Raman), due to molecular vibrations characteristic of molecules or groups of atoms. Infrared spectroscopy is commonly used for the characterization of nanoparticles of diverse nature, including metal nanoparticles and carbon nanomaterials, as well as core nanoparticles.9
In terms of spectroscopic techniques, the infrared spectral range for the near infrared is 0.9-3μm and in the mid-infrared range of 3-20μm. They provide discriminatory information due to the excitation of the vibrational and vibro-rotational transitions and bands of spectra that correspond mainly to overtones and bands of combination of fundamental transitions.9 In the specific case of silver nanoparticles, many of the techniques mentioned above can be used for the characterization of their physical and chemical properties, studies have been reported using multiple of these techniques such as AFM, TEM, DLS, for the characterization Physicochemistry of AgNPs, besides the determination of size, agglomeration, morphology and stability of AgNPs exposed to different concentrations of acid using TEM. In the specific case of AgNPs, the simultaneous determination of ionic silver and AgNP in colloidal suspensions still present an analytical challenge for most of the techniques.10
Applications of silver nanoparticles as a bactericidal agentThe silver nanoparticles (AgNP) have a great potential as an antimicrobial agent and that is why they have been used in surgical coatings, in the cosmetic and textile industry, as well as in household appliances for water treatment. According to some studies, the smallest nanoparticles exhibit a greater bactericidal action. It has been found that the silver particles that are synthesized with sodium borohydride as the primary reducing agent and trisodium citrate secondary reducing agent and as a stabilizer have the bactericidal activity which depends on the size and the dose of the nanoparticles. In a real study, they evaluated the minimum inhibitory concentration and the minimum bactericidal concentration, for this, they tested nanoparticles of 5, 7 and 10nm in different microbial strains; in the case of E. coli in the presence of 5nm silver nanoparticles. There was bacteriostatic activity (inhibiting the growth of microorganisms) in one hour, whereas in the presence of AgNP of 7 and 10nm it lasted twice as long. The fact that a smaller diameter of AgNP has a higher bactericidal activity is due to the fact that the size of the surface increases; that is, as the diameter of the particle decreases, the ratio of surface area to volume increases.11 Recently, it has been found that the silver selenide and silver sulfide nanoparticles obtained from a synthetic zeolite (A4) matrix with the formula Na12[(SiO2)12(AlO2)12] 27H2O have antibacterial properties; since in the presence of bacterial strains present inhibitory action, in fact in the gram-positive strains have greater inhibition than in the gram-negative as shown in Figure 13.12 By means of these results it can be found that the silver selenide nanoparticles have greater antibacterial activity than the silver sulfide nanoparticles. This study allows to affirm that this type of nanoparticles has a potential application in the field of biomedicine.12 In another study they also used the disc diffusion method for the determination of the antimicrobial activity of the AgNP, in this case they tested with AgNP stabilized with dextran sulfate, a variety of bacterial strains were used. According to the authors, the bacterial suspension contained 108 colony-forming units per milliliter, and for the test, the following concentrations were used: 0.25mg/mL, 0.5mg/mL, and 1mg/mL in 9-mm discs, were incubated for one day at a temperature of 37°C.13
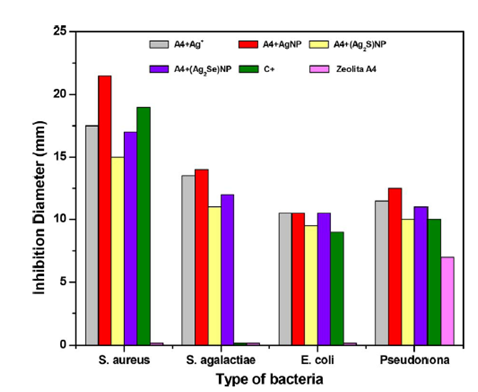
Figure 13 Results of the inhibitory action of silver nanoparticles, silver selenide and silver sulfide.12
As it is observed, in Table 3, as the concentration of nanoparticles increases, the bactericidal activity increases, since the inhibition halo is of greater diameter. Antimicrobial activity was found in the solutions with AgNP stabilized with dextran sulfate, however it expressed higher antibacterial activity for the gram positive bacteria Bacillus luteus in haus strain, followed by the gram negative Escherichia coli.13 According to the literature, the possible mechanism of the bactericidal action of AgNP is the formation of reactive oxygen species that damage microbes, DNA and proteins and the cell membrane.12 This is because nanoparticles are incorporated into the interior of the cell by diffusion, endocytosis and adhesion, there are nanoparticles that generate reactive oxygen species that oxidize double bonds of the fatty acids of the membrane, which allows it to have greater permeability and therefore is harder to obtain nutrients. Furthermore, these peroxidized fatty acids allow other free radicals to be generated, causing damage to the cell membrane. In addition, it can be due to the accumulation of silver in the membranes of bacteria that cause cell death, because silver as a cation reacts with thiol groups and proteins by inactivating enzymes that are responsible for cell metabolism.13 To determine the formation of reactive oxygen species, tests are carried out to determine if AgNP release these species, for example, in investigations 2,7-dichlorofluorescin-diacetate (DCFH-DA) has been used as an indicator, if after a time the bacteria that are in contact with the AgNP are stained there is an indicator that reactive oxygen species such as hydrogen peroxide that cause cell death were released.14 This occurs by the oxidation of the non-fluorescent DCFH to highly fluorescent (DCF+); DCFH upon entering the cell is hydrolyzed by the esterases and in the presence of hydrogen peroxide it is converted to the fluorescent form and in this way a count can be performed under a microscope.15 As shown in the following figure (Figure 14), when there is presence of AgNP in the plasmatic membranes of the bacteria, an alteration of normal physiological function occurs as it intervenes with the flow of electrons through the plasma membrane. This can generate oxidative damage, since an electron transfer to oxygen can occur to form hydrogen peroxide.15
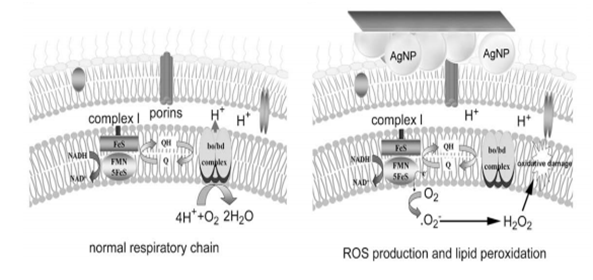
Figure 14 (A) Normal electronic transport through the respiratory chain in the plasma membrane of a bacterium in complex I. (B) Electronic transport altered through the respiratory chain in the plasma membrane of a bacterium in complex I due to the presence of AgNP.15
Concentration |
0,25 mg/mL |
0,5 mg/mL |
1 mg/mL |
||
Bacterial strains |
Gram positive |
Staphylococcus aureus |
17 |
18 |
19 |
Bacillus cereus |
16 |
18 |
19 |
||
Bacillus luteus in haus strain |
20 |
21 |
24 |
||
Bacillus subtilis |
16 |
17 |
19 |
||
Listeria monocytogenes |
16 |
17 |
18 |
||
Gram negative |
Escherichia coli |
17 |
18 |
21 |
|
Table 3 Radial diameter of inhibition zones for tested bacterial13
The size, shape, in general the morphology of the nanoparticles dependent on the specific control of factors such as reaction kinetics, temperature, pH, etc., so that the synthesis method is selective in obtaining the nanoparticle what is sought to be synthesized.
None.
None.
Authors declare that there is no conflict of interest.

©2019 Gamboa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.