International Journal of
eISSN: 2573-2838


Review Article Volume 3 Issue 3
1Chemistry Department, King Abdulaziz University, Saudi Arabia
2Chemistry Department, Assiut University, Egypt
Correspondence: Mahmoud A Hussein, Chemistry Department, Faculty of Science, King Abdulaziz University, PO Box 80203, Jeddah 21589, Saudi Arabia
Received: October 03, 2017 | Published: October 17, 2017
Citation: Albeladi HK, Al-Romaizan AN, Hussein MA. Role of cross-linking process on the performance of PMMA. Int J Biosen Bioelectron. 2017;3(3):279–284. DOI: 10.15406/ijbsbe.2017.03.00065
In order to encounter the necessities of the application, cross-linking of poly(methyl methacrylate) (PMMA) has been emerged and become an interesting issue, due to the unsatisfied results that result by the neat poly(methyl methacrylate) itself. Numerous facets make cross-linked PMMA superior and preferred than uncross polymer like the enhanced heat performance, the outstanding mechanical properties, its chemical resistance and good abrasion resistance. UV radiation, Cross-linking by free radical, small-molecule cross-linking, and condensation are discoursed concisely in the following review, also the effects of cross-linker on polymer properties are discussed. Importantly, present the review discusses the different important applications of cross-linked PMMA.
Keywords: poly (methyl methacrylate), cross-linking, thermal stability, medical applications
There is no doubt that, poly (methyl-methacrylate) (PMMA) is commercially used synthetic resin that produced via polymerization of its starting methyl-methacrylate monomer. It is a multipurpose amorphous thermoplastic polymer that is widely used in optic-electronic instruments like: surface boosted Raman scattering substrates,1 lightweight window,2 solar cells,3 light-emitting diode 4 and battery5 because of its significant characteristics such as: its exceptional optical transparency, low-priced, mechanical properties, chemical resistance, and tremendous electrical.6 Also, PMMA is used commonly in electronic, surgical and dental supplies, as it is characterized by its resistance to several inorganic reagents, acidic and alkaline solutions, non-polar solvents, and aliphatic hydrocarbons.7,8 Unfortunately, the hydrophobic nature and lack of triggered organic groups on PMMA, its low mechanical - dynamical properties under high temperature circumstances and thermal steadiness considered as drawbacks of PMMA that restricted its applications. There is an important well known method used to improve the properties of PMMA radically such as: its influence in the chemical, thermal, physical, morphological, and mechanical behavior of a polymer, which made it used potentially in different fields such as electronic applications,9 humidity sensors10 batteries.11 This method is called Cross-linking in which it tends to polymer modification by the process of interlinking the polymer chains through ionic or covalent bonds, in which it confines the polymer chains to slide past each other by impeding their free movement and produces elasticity in amorphous polymers at the same time, and this process can be carried out by using (i) chemical cross-linking or (ii) physical procedures.
Chemical cross-linking produce new polymer derivatives that completely have different physical as well as chemical properties. In most cases, chemical modifications enhance the total performance of PMMA and lead to unexpected new type of application that can be utilized for the new products. Table 1 summarize the most promoted characterizations after cross-linking occurs.10,12-17
Characteristic of PMMA |
Change After Cross-Linking of PMMA |
Ref |
Thermal stability |
Greatly improved |
|
Tensile strength |
Increase |
|
surface hardness |
Increase |
|
Mechanical stability |
Greatly improved |
|
flame retardant |
Increase |
|
Elasticity |
slightly increase |
|
solvent resistance |
Increase |
|
chemical resistance |
Increase |
Table 1 The most significant poly(methyl methacrylate) characterization changes after cross- linking process
Cross-linking by means of free radical polymerization
In order to attain chemically cross-link polymers, there are techniques used such as suspension, emulsion, and dispersion polymerization techniques in which suspension polymerization technique is used preferably.18 In the occurrence of an initiator and heat Free-radical polymerization takes place. The synthesis of GDMA-PMMA resin is carried out by the cross-linking process of GDMA with methyl methacrylate (MMA) through free radical polymerization, in which the GDMA-cross-linked PMMA polymer is acquainted with being an effectual sustenance for solid phase peptide production as illustrated in Figure 1. The new flexible GDMA-PMMA resin can be designed for the synthesis of hydrophobic peptides in very high yield and transparency.15
Cross-linking Through condensation reactions
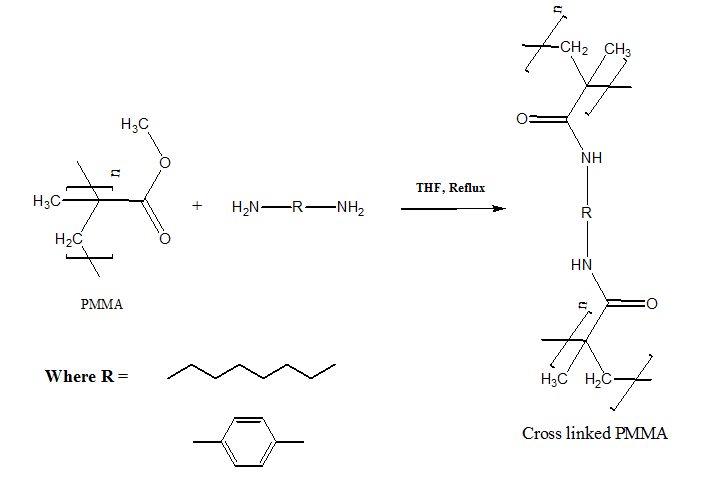
This form of polymerization takes place in the presence of heat, catalyst, or both. According to the bridging groups formed during polymerization, the polymers obtained by means of this method may be degradable19 or non-degradable. PMMA with diamine compounds can be produced by condensation reactions among the carbonyl group (>C=O) with Amine group (-NH), These reactions may be used for to improve PMMA properties mainly thermal steadiness and texture behavior by means of normal chemical action by the use of aliphatic and aromatic diamines in several ratios as cross-linking agents as shown in Figure 2.12
Small-molecule cross-linking
Some Small-molecules considered as a cross-linker and used potentially to obtain cross-linked polymer such as: formaldehyde, glutaraldehyde, potassium dichromate, osmium tetroxide, and potassium permanganate, other than the multifunctional (bi, tri, or tetra) cross-linking agent.20,21 This technique is used mostly for the polymers containing, carboxylic acid, hydroxyl and amine grounded functionality, and it is used extensively like addition or condensation polymerization by free radical polymerization polystyrene (PS) and PMMA amide functionalized were produced. For example PMMA polymer was efficiently cross-linked with a small molecule cross-linker (1,3,5-tris(2-propynyloxy)benzene) by means of a thermally triggered reaction at 100 °C as displayed in Figure 3. Good solvent resistant has been shown through the cross linked PMMA thin film and displayed significantly enhanced dielectric properties associated with uncross-linked polymer, which makes this cross-linking technique companionable with plastic substrates for malleable electronic applications.9
Cross-linking by means of radiation
Radiation treatment such as the gamma exposure, proton, electron, implantation and ion irradiation 22-25 can alter the polymeric properties; in which radiation induced cross- linking of polymers have extensive variety of uses on the profitable levels like: rubber tires, wires, cross- linked cables, development of cross- linked silicon carbide fibers and polymer recycling. The irradiation environments for PMMA thin films to induce cross-linking in the polymeric chains have been improved by irradiating it with 2 MeV proton beam while preserving the exposed area free of blister.26 Figure 4 shows an schematic illustration summarize the most important methods of cross-linking for PMMA polymer. In general, a brief comparison between the main four types of cross-linking processes is displayed in Table 2. The present comparison is mainly based on variable items including: number of steps, cross-linking & curing mechanism, curing temperature, mechanistic approach, curing time, equipment cost, bond strength, and degree of cross-linking.
Cross-Linking Process |
Free Radical Polymerization |
Condensation Reactions |
Small-Molecule |
Radiation 27 |
No. of steps |
One step |
One step |
Two step |
One step |
Cross-linking mechanism |
Free radical |
Grafting |
Free radical |
|
Curing mechanism |
- |
Condensation reaction |
Condensation reaction |
- |
Curing temperature |
80 °C |
65-67 °C |
100 °C |
Room temperature |
Mechanistic Approach |
Monomer/Monomer |
Polymer/Monomer |
Polymer/Monomer |
|
Curing time |
less |
High |
Very high |
Very low |
Equipment cost |
Low |
Low |
Low |
High |
Bond strength |
Strong |
Strong |
Strong |
Strong |
Degree of cross-linking |
Degree of cross-linking is |
Degree of cross-linking is |
Degree of cross-linking is |
Table 2 A Comparison between four main types of cross-linking processes
Cross-linking processes for PMMA have been widely utilized in order to overcome the significant week points in leaner PMMA such as rigidity, mechanical strength, strain modulus, and stiffness. In addition to thermal stability as well as morphological behaviors which are completely affected by cross-linking process. The cross-linked product is completely affected by the type of cross-linker and its concentration. Properties like thermal degradation, glass transition, particle size, pore size, pore volume, surface area, and swelling are totally changed after cross-linking occurred. The most important cross-linker types are hydrophilic, hydrophobic, rigid, or flexible. Table 3 shows the most important monomers and their common abbreviations that predominantly used as cross-linkers or cross-Linking agents in such cross-linking process for PMMA.
Monomer |
Abbreviation |
Chemical Structure |
Ethyleneglycol dimethacrylate |
EGDMA |
|
Trimethylolpropane triacrylate |
TMPTA |
|
Hexamethyleneglycol dimethacrylate |
HMGDM |
|
Tetraethylene glycol dimethacrylate |
TEGDMA |
|
Divinyl benzene |
DVB |
|
1,6-diaminohexane |
DAH |
|
p-phenylenediamine |
P-PHDA |
|
Table 3 The most common cross-linking monomers used in cross-Linking agents of PMMA
Medical applications
In joint replacement surgery PMMA, based cements are used extensively in bone cements. However, there are some drawbacks in the usage of these cements, as the clinical achievement percentage is properly high. It has been stated by Deb, S. and B. Vazquez, that dimethacrylate cross-linking agents was added to the monomer stage with the purpose of producing a cross-linked matrix, along with using barium sulphate as a radiopaque agent, and by this, the results suggest that the mechanical properties can be enhanced or reserved by means of addition of those cross-linking agents.13 One of the predominantly used polymers in dental instruments is PMMA as it is used extensively for fabricating dentures, in which the denture base polymer are multiphase polymers made of PMMA beads and monomers enclosing cross-linking agent, as there are lots of cross-linking agents have been used as a factor of PMMA denture-base material.30,31 The outcome of the point of cross-linking on swelling and drug swelling and drug release behavior of poly (MMA-coitaconic acid) [P (MMA/IA)] hydrogels for site specific drug distribution has been presented by Khalid S et al.32 putting in consideration that the Cross-linking percentage is one of the most significant factors that affect the swelling of the hydrogels, in which any increase occur in the degree of cross-linking of the system will effect in a stronger gel, also it was detected that the MMA/IA polymer is appropriate for distributing the drug above pH 6, whatever the degree of cross-linking.32 In literature there is a work presented by Nein et al.33 stating that bone cement is produced for drug release with the ability of control, and an increase in both the drug release rate and total release amount of the drugs, devoid of losing the mechanical characteristics of the cement, and that was accomplished by acquainting with cross-linked PMMA-acrylic acid sodium salt particles into the bone cement, that intensify the hydrophilicity of the cement for the easy channel of liquids.33
Energy storage
Krebs H et al.34 presented a series of methacrylate-cross-linked polymers as impending polymer electrolytes for energy storage application, in which two series of porous thin films have been equipped using precipitation polymerisation of MMA with methacrylate ester cross linkers of altered spacer lengths, and the result was that both series of polymers exhibited high thermal steadiness corresponding with their molecular structure and the thin polymer electrolyte films turn out to be harder and more elastic by means of condensed crosslink density and increased spacer length of the crosslinking agent. As a result of the grouping of properties makes these thin films encouraging applicants for further improvement of directly process able, electrolyte filled strainers for Li-ion membranes and permit further development and assessment.34
Transistors
Yun Y et al.35 reported in their work that the physical cross-linking of the PMMA gate dielectric can be used efficiently to create thin-film transistors with extraordinary carrier flexibility and functioning at low voltages.35 Table 4 summarizes the important as well as variable applications for PMMA cross-linked derivatives.36-44 The present summary deals with critical points of view such are method of preparation, cross-linking agent, and advantages.
Substrate |
Preparation Method |
Cross -Linking Agent |
Advantages |
Applications |
Ref |
PMMA |
Precipitation polymerisation |
Methacrylate ester crosslinkers of |
Their unique set of properties of |
Energy storage |
|
Poly(sodium pstyrenesulfonate)/PMMA |
In situ cross-linked polymerization |
N,N´-methylenebis (acrylamide) |
More than 90% of cationic dye was |
Wastewater treatment |
|
PMMA |
Spin-coating and subsequent |
- |
can be operated at low voltages |
Transistors |
|
PMMA/n-hexadecane microcapsules |
Emulsion polymerization, |
Allyl methacrylate and ethylene |
The enthalpies of microencapsulated |
Fabrics |
|
CD-MA PMMA nanogels loaded with |
Radical precipitation Polymerization |
α-, β-, and γ-cyclodextrin Methacrylate |
The controlled and safe use of high |
Medical and Dental applications |
|
TIPS-tetracyanotriphenodioxazine |
Irradiation |
1,6-bis(trichlorosilyl)hexane |
linear regime field-effect mobility |
Flexible applications |
|
PS/PMMA |
1,3,5-tris(2-propynyloxy)benzene |
Good solvent resistant significantly |
Electronic applications |
||
PMMA |
Irradiation |
- |
PMMA was found to have the largest |
Lithography |
|
(mPEG-PMMA-SS) micelles |
Atom transfer radical |
Disulfide |
Biocompatible and nontoxic up to a |
Drug release |
|
[P(MMA/IA)] hydrogels |
Solution polymerization |
Ethylene glycol dimethacrylate |
MMA/IA polymer is appropriate for |
Drug delivery |
|
PMMA/ barium sulphate |
Dimethacrylate |
Good mechanical properties |
Bone cements |
||
PMMA powder (CPM-PMMA) |
Suspension polymerization. |
Ethylene glycol dimethacrylate, |
Good mechanical properties |
Dental applications |
Table 4 Advantages and important applications of cross-linked PMMA derivatives
Cross-linked PMMA has a significant purpose as it affords products that have not only the characteristics of systematic polymer but also exceptional surplus ones, which expedite its new application in capricious arenas of industry. This review has concisely talked over the influence of Cross-linking Process on the Performance of Poly(methyl methacrylate). The most common for types of cross-linking processes are compared to each other according to variable conditions including: no. of steps, cross-linking & curing mechanism, curing temperature, mechanistic approach, curing time, equipment cost, bond strength, and degree of cross-linking. In addition gives a general overview to the various techniques of cross-linking, effects of cross-linker on polymer characteristics, and finally brief overviews for its important as well as famous applications.
None
The author declares no conflict of interest.

©2017 Albeladi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.