International Journal of
eISSN: 2573-2838


Mini Review Volume 4 Issue 6
Department of Biomedical Engineering, Sheikhbahaee University, Iran
Correspondence: Hamidreza Shirzadfar, Department of Biomedical Engineering, ?Sheikhbahaee University, Isfahan, Iran
Received: September 26, 2018 | Published: November 14, 2018
Citation: Shirzadfar H, Khanahmadi M. Review on structure, function and applications of microfluidic systems. Int J Biosen Bioelectron. 2018;4(6):263. DOI: 10.15406/ijbsbe.2018.04.00137
Microfluidic is the use of special properties of fluids in very small volumes such as Picoliter, Nanoliter or Microliter. The flow of these fluids is regulated in the micro channels. These canals are made in very small dimensions, and the material of these microchannels is basically polydimethylsiloxane (PDMS). This technology is widely used in medical and tissue engineering and research and diagnostic fields. The microfluidic device is made up of glass, silicon or the like, in which the micro channels are inserted into the chip. Different methods such as photolithography and so on are used to make these types of chips, each with many advantages and disadvantages. The microfluidic device has several components, each of which has a special function. The microfluidic systems have many advantages that make this technology much to be considered.
Keywords: Microfluidic, PDMS, micro channel, lab on a chip, photolithography
Microfluidics is made on a very small scale, which can be used in very small volumes of fluids. In this system, fluids are contained within the microchannels, which perform various operations on the fluids.1,2 Inside microfluidic devices in addition to micro channels, other components including pumps, valves and mixers are available.3 Several types of microfluidic penetrate into these micro channels, and then some of these fluids are stored in the micro channels. Then, these fluids are combined with the mixers and create a specific reaction. Finally, the final product of this reaction will come out of the machine. The function of this device can be checked by ultraviolet microscopes, etc.4,5 Microfluidic systems have vast applications that have widespread use of the system in medicine and biology, as well as in research and laboratory. The technology of lab on a chip (LOC) is one of the applications of these systems that part of the chip acts as a part of the lab. The benefits of this technology include high sensitivity, isolation, reduced time and cost, high separability and excellent detection (Figure 1).6–8
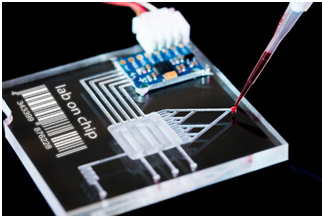
Figure 1 The example of lab on a chip.8
Several factors have contributed to the emergence of microfluidic system, the most important of which is the molecular biology and the use of genomes. Microelectronics also has made a lot of progress in this systems.9 Initially, glass and silicon were used to make chips, but then better materials were replaced. Today, Siloxane and PDMS (polydimethylsiloxane) are used to make chips more beneficial than previous ones (Figure 2).10–12 These types of chips are very permeable to many gases, which are very important for the evaluation of the cells of the living creatures. The surface properties of this kind of chips can be changed. These materials are very transparent, and it is easy to check the behavior of fluids by optical microscopy, as well as biocompatible and non-toxic. The type of fluid movement in the micro channels is different. In some cases, it is possible that two types of fluids injected into the micro channel are not combined, which should result in different ways to correct these things. On different scales of microfluidics, the type of motion and flow of fluids are different, which can be used for different applications. By using electro-osmotic flow in micro channels, separation and isolation can be increased in this system.13–15 The microfluidic device consists of several components, such as valves and pumps, and mixers, each of which has a special function. The valves in the microfluidic systems guide the fluids on the micro channel path. Mixers are used to combine different types of fluids, which ultimately carry these compounds by pumps.16 It is very important to make chips, the location of the channels, a special chemical pattern and the physical form of these chips. Some parts of these micro channels should be absorbent so that some materials are stored in these areas, and some places should also prevent material from joining.17
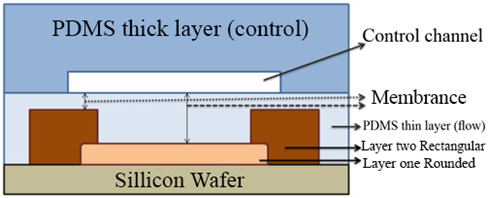
Figure 2 Tape of chips with PDMS.12
There are several methods for making microfluidic chips. The photolithography method is one of the basic methods for making this chip. In addition, Self-assembled monolayers are used to make chips.18,19 The microfluidic system is very useful for its specific features, including very low volumes, different fluid movements, and electro-osmotic flow properties and laminar flows in various fields. The microfluidic system can be very useful for cellular studies, and these chips are easily adapted to cellular environments and can be placed within cells and can be easily controlled and monitored around the cell. To cultivate and study stem cells, a microfluidic system can be used that is more beneficial than other methods, and is carried out in a micrometric environment, saving both time and cost, as well as stem cells throughout The cultivation will remain healthy.20,21 Electrochemical Impedance Spectroscopy can also be used to study the cell. The potential applied to the system creates a sinusoidal current, which ultimately results in this impedance in terms of current and voltage. Once the impedance spectrum is obtained, the cell components can be displayed.22,23 Using the microfluidic system, neutrophils can be traced to some of the reactions and follow. Basically, this kind of system has two inputs that are input from each input of the HBSS (Hank's Balanced Salt Solution) buffering solution. The solutions are then continuously separated and then combined with a mixer. At the end of the system, these two channels merge together and form a broad channel. This long channel is used to examine neutrophils24,25 (Figure 3). The features of the microfluidic system can be used to investigate injuries to neuronal axons. Therefore, the diseases associated with axons can be tracked and carefully studied. Microfluidic are very useful in the diagnostic field and can be used to identify various diseases26 (Figure 4). The microfluidic system can be used to test the nucleic acids and DNA. In this system, there is an input channel that enters the blood into the channel and the necessary processes, including separation and dilution, are performed on the blood sample.28
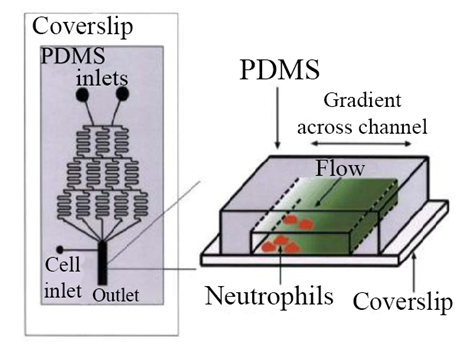
Figure 3 Evaluation of neutrophil movement in the microfluidic system.25
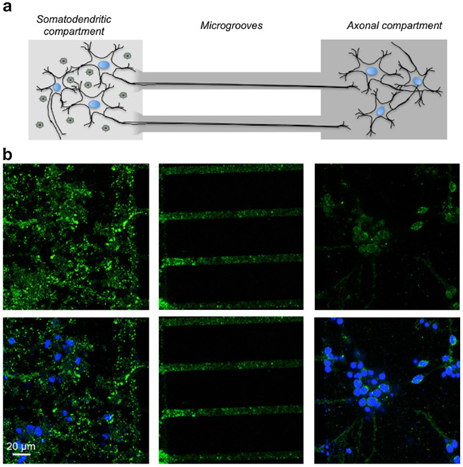
Figure 4 Study of neuronal axons in the microfluidic system.27
The microfluidic system is very useful in the laboratory and in the research process. These chips can be used as part of the test site, which is called the technology of lab on a chip (LOC). This technology has many advantages, including reducing human error, reducing costs for expensive samples, reducing energy, very low volume and increasing sensitivity.29 This technology can be used to treat cancer, which can be used with Drug delivery.30 LOC is used to deliver the drug to cancer cells in such a way that healthy cells do not get harmed. This technology is more likely to reduce the pain associated with cancer treatment so that it can be treated noninvasively and also has definitive cancer treatment. Also important LOC’s applications can be used to diagnose and control the AIDS virus.31 The microfluidic system can be used to check blood glucose levels. Microfluidic can be used to analyze blood and measure some of the substances in the blood such as glucose. New methods can be used to detect non-invasive glucose by using optic sensors integrated with microfluidic. The microfluidic chip can be used as a platform for optical sensors that increase the sensitivity of the sensor. Using this method, these sensors are very small and portable.32,33
As stated in this paper, microfluidic systems are built on very small scales, which have made this system more widely used in various fields such as industry, medicine, molecular biology, laboratory, etc. This kind of system can be used to check the cells and surrounding areas of these cells. These chips are biocompatible and can be used to check the cells of the living creatures. This system is very useful in the field of diagnosis. The microfluidic device reduces time and cost and also increases sensitivity and separability.
None.
Authors declare that there is no conflict of interest.

©2018 Shirzadfar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.