International Journal of
eISSN: 2573-2838


Research Article Volume 7 Issue 4
1Department of Chemistry, Ain Shams University Cairo, Egypt
2Department of Polymers and Pigments, National Research Centre, Egypt
Correspondence: Afify MF, Department of Chemistry, Faculty of Science, Ain Shams University Cairo, Egypt
Received: August 21, 2021 | Published: August 31, 2021
Citation: Afify MF, Mekewia MA, Abd El Ghaffarb MA, et al. Poly (butyl acrylate- methyl methacrylate- acrylamide)/ bentone 34 nano composites for electrical insulation. Int J Biosen Bioelectron. 2021;7(4):105-111. DOI: 10.15406/ijbsbe.2021.07.00222
Poly (butyl acrylate- methyl methacrylate-acrylamide)/nano bentone 34 composites were prepared through bulk polymerization in presence of BPO (benzoyl peroxide) as initiator. The (BA/MMA/AAm) terpolymer/nano bentone 34 composite sheets were molded at 180°C prior subjecting to the specific mechanical and electrical characterization. Structural, physical and thermal validations were conducted through GPC, FT-IR, HRTEM and TGA classified techniques. The results of the electrical resistivity and mechanical properties aptitudes signpost step towards a new generation of insulators for electrical cables according to IEC and BS standards.
Keywords: poly (butyl acrylate-methyl methacrylate-acrylamide) bentone 34, insulators for electrical cables
Electric power cable is an assembly of two or more electrical conductors; each is insulated and held together with an overall sheath which is used for the transport of electrical power,1 Rubber cable insulation lasts usually for 25-30 years and remains soft and pliable even when the temperature is low. PVC insulation, on the other hand, becomes stiff, due to weathering conditions, burning PVC emits toxic dioxin.2 Poly (methyl methacrylate) is the polymer product of methyl methacrylate monomer, it is a clear, colorless polymer that is produced by free‐radical polymerization of methyl‐ methacrylate in mass or suspension polymerization.3 PMMA is a linear thermoplastic polymer that acquires high mechanical strength, high Young's modulus and low elongation at break. It is one of the hardest thermoplastics and of anti-scratch feature. PMMA exhibits low moisture and water absorbing capacity, due to which products made have good dimensional stability.3 PMMA, is one of the most widely used type of acrylate polymers due to its high clarity and outstanding outdoor weathering resistance, easy handling and processing, and low cost. However, PMMA usually behaves in a brittle manner when loaded, resulting in its restricted application. The first common solution is the copolymerization of MMA with other monomers that are of glass transition temperature (Tg) below room temperature, e.g., butyl acrylate polymers (PBA)4,5 for more applicable molded articles.
More interest in using polymer layered silicate (PLS) was developed successfully in many industries and especially in the automotive industry, since the first invention was made by Toyota car manufacturers group during 1980’s. Due to its high strength and thermal resistance, On the other hand, great fund is directed nowadays towards food chemistry and health care, which makes clay Nano composites a favored candidate due to its barrier properties which would prevent penetrations of moisture or harmful gases.6 Dispersion of clay particles into the polymer matrix is the most challenging issue in all fabrication techniques. As poor dispersion will lead to agglomerated spots, where many clay particles are stacked together and will accordingly act as a crack propagation spot, rather than being an enhancement, when mechanical load is applied. One of the major problems is the difference in nature between the organic polymer and the silicate layers. While clays are hydrophilic in nature and highly susceptible to swell water, polymers are hydrophobic in nature, which prevents bonding and the ability of the polymer to penetrate through stacked silicate layers.6 The surface modification of clay layers by organic material enhance clay dispersion in the polymer matrix , which can be achieved through a cation exchange process by the replacement of sodium and calcium cations present in the inter layer space or clay galleries by alkylammonium or alkylphosphonium cations.7 The objective of this work is to improve the physical, electrical, thermal and mechanical properties of P (BA/MMA/AAm) terpolymer by addition of organically modified nano clay (bentone 34) to design a valid, compatible and opportune electrical insulation that satisfies measurable specified properties of cable insulators.
Butyl acrylate monomer (BA) provided by Sigma Aldrich Company, acryl amide monomer (AAm) provided by Merck-Schuchardt Company and methyl methacrylate (MMA) by Alfa Aesar Company. AAm monomer was used without further purification while MMA and BA monomers were activated by shaking, several times, with 5% NaOH solution to remove the stabilizer and then washed by distilled water several times until neutrality. Freshly inhibitor-free monomer was then distilled under vacuum and finally kept under Na2SO4 anhydrous. The refractive index of the inhibitor-free monomers was measured to affirm its purity before use. Benzoyl peroxide as initiator was provided by Merck-schuchardt. Bentone 34 (organically modified nano clay) provided by ELEMENTIS specialties, USA was used at different ratios.
The polymerization process
The polymerization reaction was done by mixing of 50% of inhibitor-free methyl methacrylate, 47.5% butyl acrylate, 2.5% acrylamide monomers in dry glass ampoules. BPO initiator, was then added at 0.6% by weight, different weight percent of bentone 34 were added as given in table 1.The glass ampoules were sealed under flow of purified and dry nitrogen gas, then placed in water bath at 60-700C for 4 hours, then left to cool to ambient temperature and finally broken to release its contents that molded at 1800C by laboratory press.
Monomer % (by weight) |
Bentone 34% (by weight) |
||
BA |
MMA |
AAm |
0 |
47.50% |
50% |
2.50% |
1 |
47.50% |
50% |
2.50% |
3 |
47.50% |
50% |
2.50% |
5 |
47.50% |
50% |
2.50% |
7 |
47.50% |
50% |
2.50% |
10 |
Table 1 Formulation of P (BA/MMA/AAm) and composites
The molecular weights of the prepared terpolymers in absence and presence of bentone 34 were determined using Gel Permeation Chromatography using tetrahydrofuran (THF) as the mobile phase. Table 2 shows that the weight average molecular weight (Mw) and number average molecular weight (Mn) in absence of bentone 34 are 0.032026×105 and 0.029464×105 respectively with a degree of Polydispersity (PD) @1.0869 which confirms good molecular weight distribution. When adding bentone 34, to the terpolymer , both the weight average and number average molecular weights start to increase as the concentration of bentone 34 increases up to 10% at distinguished values of 4.5465×105 and 2.1647×105, respectively, Figure 1A-1C, i.e. in this case bentone 34 increase the propagation steadily.
|
Bentonite (Wt. %) |
𝑴𝒘 |
𝑴𝒏 |
PD |
|
zero |
0.032026×105 |
0.029464×105 |
1.0869 |
|
1% |
2.3413×105 |
0.92044×105 |
2.5437 |
|
3% |
3.2853×105 |
1.1925×105 |
2.755 |
|
5% |
3.3433×105 |
1.5238×105 |
2.194 |
|
7% |
4.044×105 |
2.0919×105 |
1.933 |
|
10% |
4.5465×105 |
2.1647×105 |
2.1003 |
Table 2 Effect of bentone 34, on the weight average molecular weight, the number average molecular weight and the Polydispersity of P(BA/MMA/AAm)
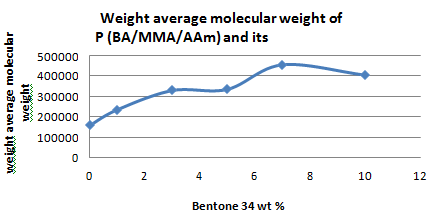
Figure 1A Effect of bentone 34 on the weight average molecular weight of P (BA/MMA/AAm) and its composites.
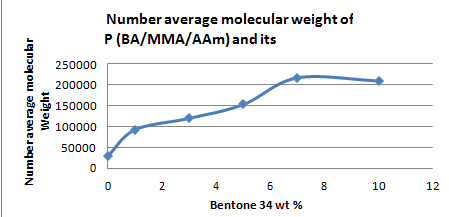
Figure 1B Effect of bentone 34 on the number average molecular weight of P(BA/MMA/AAm) and its composites.
Chemical structural characterization (FT-IR spectroscopy)
IR Spectra of the prepared terpolymer and composites were obtained using FT IR Nicolet 6700 spectrophotometer. IR Spectrum of bentone 34 gives a good confirmation of its structure as shown in Figure 2A. The absorption bands at 3630cm−1 and 916cm−1 are attributed to Al−OH stretching and bending vibration, while the band at 625cm−1 can be attributed to Si–O–Al bond. The two absorption bands observed at 436cm−1 and 522cm−1 can be related to the low frequency Si−O bending vibration. For the quaternary ammonium salt, two characteristic absorption bands at 2850 and 2920cm-1 can be assigned to the C−H stretching vibration of -CH3 and -CH2 groups, respectively, and the -C−N stretching vibration band at 1045cm-1 also assigned to quaternary ammonium salt, this is in addition to the absorption corresponding to 𝜈(𝑁+𝐻𝐶𝑙−) band. FT-IR spectrum of the prepared terpolymer was illustrated in Figure 2B. The spectrum exhibits the characteristic absorption band of -C=O stretching vibration at 1730cm-1 while the absorption bands at 2960, 2875 cm-1can be attributed to the -C−H stretching vibration of -CH3 and -CH2 groups. The -C−H in-plane bending vibration of -CH3 and -CH2 groups appeared at 1387, 1450cm-1. This is in addition to -C−O−C single bond stretching vibration and single bond deformation vibration bands appeared at 1260cm-1 and 844cm-1 respectively which are characteristic for PMMA and PBA and that at 3400 cm-1refer to the −NH stretching vibration broad band, characteristic for PAAm. In the presence of bentone 34, the- C−N stretching vibration band at 1045cm-1 seems to disappear while the same characteristic bands of the terpolymer still appear, in addition to absorption corresponding to (𝑁+𝐻𝐶𝑙−) band as shown in Figure 2C.

Figure 2A FT-IR spectrum of bentone 34, 2B: FT-IR spectrum of P (BA/MMA/AAm), 2C: FT-IR spectrum of P (BA/MMA/AAm)/ bentone 34 composite.
Microstructure investigation (HRTEM imaging)
High resolution Transmission electron microscopy (HRTEM) is the most commonly used tool to characterize the microstructure of nanocomposites. Figure 3A illustrate the TEM micrographs of Bentone 34, where firmly and reportedly a regular sheet structure with particle sizes in nano scale Upon polymerization in presence of the bentone34, the polymers were observed finely intercalated in the layers of it as shown in Figure 3B, where the black lines are the cross section of aluminosilicate platelets, the platelets can be seen as a single and uniformly dispersed, this shows that the monomers had polymerized in the bentone 34 laminar and formed inorganic-organic hybrid. Such intercalation could be principally assigned to the improvements observed to both the mechanical and electrical properties of the polymer composite in addition to its thermal and anti-degradation aspects.
Thermal studies
The thermal stability of the terpolymer in absence and presence of bentone 34 was investigated using TGA analysis under nitrogen gas at a heating rate of 10oC/min over a temperature range from room temperature up to 500oC.The terpolymer showed a weight loss 0f ≅1.35% residual weight at elevated temperature (>400oC) and the first derivative (DTG) showed that this terpolymer was degraded at nearly 396oC, Figure 4A, In presence of 5 and 10wt.% bentone 34 the residual weights increased up to 5.87 and 9.35% with an inflection point at 395 and 399oC respectively, Figure 4B & 4C. By calculation the degradation temperature at 10% and 50%wt. loss from TGA curves we can concluded that bentone 34 enhance the thermal stability of all terpolymers at the earlier stage of decomposition especially with 10wt. % from bentone 34 as illustrated in table 3, this observation can be attributed to the presence of high wt.% from bentone 34 which is highly thermal stable inorganic material which act as barrier for heat transmission and preventing the out diffusion of volatile decomposition product,8 but in the final stage of decomposition there is no observation for an advantageous effect of bentone 34 on the degradation of the prepared terpolymers which indicate an absence of bentonite (bentone 34) intrusion or conjugation to the chemical structure of either polymer in the composite. Such results indicate clearly that the role of bentone 34 material is merely a supporting agent towards the improvement or depreciated specific physical and mechanical properties and the role played only during the propagation and termination of polymerization steps.
|
Sample number |
Td10% 0C (degradation temperature at 10 % weight loss) |
Td50% 0C (degradation temperature at 50 % weight loss) |
|
Terpolymer II |
350 |
396 |
|
Terpolymer II / bentonite 10 %) composite |
362 |
399 |
Table 3 Residual weight and thermal degradation parameters of P(BA/MMA/AAm) and its composites
Mechanical properties validation
The mechanical properties of polymeric materials are most important parameters that determine the capability of material mold in suitable shape which induce in turn suitable specific and related features, it is well known that the mechanical properties of nanocomposites based on layered silicates are typically enhanced relative to those of the virgin polymer The tensile strength (T) and elongation at break (E) for different terpolymer sheets were measured according to DIN 53504 S2 – Traction9 using an electronic Gibitre tensile testing machine (tensor check profile- Italy) with a speeding rate of 50 mm/min and maximum load 20K.N. The tensile strength and elongation at break for the prepared terpolymer and composites have been measured and are given in Table 4 and Figure 5A & 5B. As noted in Table 4, The tensile strength of the prepared terpolymer is 19.8N/mm2 with elongation at break of 205%. This is indicating that the prepared terpolymer is suitable to produce polymeric material to be used as a cable insulators or sheathing according to the standard cable specification, BS 7655,10 BS 674611 and IEC 60502.12 In the presence of bentone 34 the tensile strength values fluctuate up and down depending on the addition wt. %, while its elongation at break decreased gradually, this improvement in the mechanical properties at low bentonite wt. % may be attributed to a good dispersion of the filler in the matrix and electrostatic attractions between polymer chains and surface of silicate layers. While at high bentonite concentration, it is not very well dispersed and exists as larger particles in the matrix which decrease the cohesive forces between polymer chains.
|
Bentone Wt. % |
(Terpolymer / bentone 34) composites |
|
|
Tensile strength (N/mm2) |
Elongation at break (%) |
|
|
zero |
19.8 |
205 |
|
1% |
20.5 |
201 |
|
3% |
20.3 |
203 |
|
5% |
21.8 |
198 |
|
7% |
20.7 |
172 |
|
10% |
22 |
170 |
Table 4 Effect of bentone 34 on the mechanical properties of P (BA/MMA/AAm)
Shore hardness D
Hardness is a composite property combining concept of resistance to penetration, scratching and so on. Shore hardness D, is a measure of the resistance of hard polymer material to penetration of a spring-loaded needle-like indenter. Shore hardness value may vary in the range from 0 to 100. The maximum hardness value 100 corresponds to zero penetration while zero value corresponds to minimum shore hardness (2.5-2.54mm penetration). Shore hardness D of the prepared terpolymer and composites were measured according to ISO 86813 on Multi-unit hardness tester from Gibetre – Italy. By measuring the shore hardness of the prepared terpolymer and its composites, Table 5 & Figure 6, we get that; the prepared terpolymer gives a shore hardness of 56.2 which is acceptable for using this material as sheathing materials according to the cable specification number H D 620.14 Also, the addition of bentone 34 improves a shore hardness of these blends and this improvement increased as the concentration of bentone 34 increased. Such observation indicates that the additions of bentone 34 silicate particle were not permitted to penetrate within the polymeric chains to influence the alteration of its stiffness.
|
Bentone wt. % |
Terpolymer composites |
|
zero |
56.2 |
|
1% |
56.8 |
|
3% |
57 |
|
5% |
57.1 |
|
7% |
57.6 |
|
10% |
57.8 |
Table 5 Effect of bentone 34 on shore hardness D after 15 second of P (BA/MMA/AAm)
Density measurements
Density for the prepared terpolymer and composites were measured according to ASTM D 792-9815 using Metller Toledo Model: - XP 204 Switzer land. The density measurements given in Table 6 & Figure 7. As shown, the density increased gradually as the percentage bentone 34 increased. So, according to cable specifications (ASTM D1248-98),16 these terpolymer and its composites can be used as sheathing or insulators for electric cables.
Bentone wt. % |
Terpolymer composites |
zero |
1.12 |
1% |
1.128 |
3% |
1.135 |
5% |
1.14 |
7% |
1.148 |
10% |
1.16 |
Table 6 Effect of bentone 34 on the density (g/cm3) of P (BA/MMA/AAm)
Electrical properties
The resistance of most polymers to flow of direct current is very high and conductivity probably results from the presence of ionic impurities whose mobility is limited by the high viscosity of the medium. The electrical property in term of volume resistivity and electric capacity is a one of the most important parameter for the polymeric material especially if it is used as an insulator or sheathing for the electrical cables.
Volume resistivity
Volume resistivity is an intrinsic property that quantifies how strongly a given material opposes the flow of electric current. A low resistivity indicates a material that readily allows the flow of electric current.
Electric capacity
Electric capacity is defined as the ability of a body to store an electrical charge. So, a material with a large capacity holds more electric charge at a given voltage than one with low capacity. The volume resistivity and electric capacity of the prepared terpolymer and its composites were measured and are given in Table 7 & Table 8 and Figure 8A & 8B. As shown, the volume resistivity and electric capacity of the prepared terpolymer and its composites are acceptable according to cable specifications data, IEC 60502 and ASTM D1248-98. Upon addition of Bentone 34 to the terpolymer improvement to both the volume resistivity and the electric capacity properties were observed. This behavior can be attributed to the presence of nano filler materials may affects the space charge accumulation in polymer matrix.
|
Bentone wt. % |
Terpolymer composites |
|
zero |
1.0x1014 |
|
1% |
2.0x1014 |
|
3% |
2.5x1014 |
|
5% |
2.94x1014 |
|
7% |
4.0x1014 |
|
10% |
5.6x1014 |
Table 7 Effect of bentone 34 on the volume resistivity (Ω cm) of P (BA/MMA/AAm)
|
Bentone wt. % |
Terpolymer composites |
|
zero |
45 |
|
1% |
62 |
|
3% |
65 |
|
5% |
78 |
|
7% |
90 |
|
10% |
73 |
Table 8 Effect of bentone 34 on the electrical capacity (PF) of P (BA/MMA/AAm)
Melt flow index
Melt flow index is a measure of how many grams of a polymer flow through the die in ten minutes. It is an important parameter for the fabrication of polymer especially if it is used as insulator or sheathing for the electric cables. The melt flow index (MFI) of the prepared terpolymer and composites was measured according to ASTM D128317 using ZWICK/Roell – Germany The melt flow measurements were given in Table 9 and Figure 9. The prepared terpolymer and its composites can be used as sheathing or insulating material according to ASTM D 1248- 98 categories 3, 4. Also, the presence of bentone 34 material in the terpolymers gives the same trend with a little decrease in the melt flow index. This can be attributed to the disturbance caused by the presence of Bentone 34 in the polymer matrix.
Bentone wt. % |
Terpolymer composites |
zero |
0.7 |
1% |
0.66 |
3% |
0.62 |
5% |
0.2 |
7% |
0.18 |
10% |
0.14 |
Table 9 Effect of bentone 34 on the melt flow index (g/10 min) of P (BA/MMA/AAm)
The main aim of the research topic was to elucidate acrylate based terpolymer composites to suite the function of electric cables insulators as possible match in its physicochemical and mechanical properties. Poly (BA, MMA, and AAm)/bentone 34 blend composites at varying concentration and bentone 34 loads were prepared to satisfy standards required for such applications. Molecular weight measurements data indicated that both the weight average and number average molecular weights in addition to the degree of dispersity for all prepared terpolymer composites improved in the presence of Bentone 34, due mainly to a probable role played that might had affected in the propagation step which in turn is reflected on the molecular weight parameters of the prepared terpolymers and their composites. As noticed, the presence of acrylamide monomer during the bulk polymerization process produced more ordered polymer. The following conclusions of bulk polymerization of some acrylic monomers in presence of organically treated bentonite (bentone 34) and its applicability as a cables insulator are summarized as follows:
None.
The authors declare that there is no conflict of interest.

©2021 Afify, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.