International Journal of
eISSN: 2573-2838


Research Article Volume 1 Issue 1
1Laboratoire de G nie Enzymatique, Universit Claude Bernard Lyon 1, France
2Joint Research Centre, Institute for Health and Consumer Protection- Ispra, Italy
Correspondence: Christophe A Marquette, Laboratoire de G nie Enzymatique, Membranes Biomim tiques et Assemblages Supramol culaires- Institut de Chimie et Biochimie Mol culaires et Supramol culaires- Universit Claude Bernard Lyon 1, France, Tel +33663886769
Received: September 30, 2016 | Published: November 2, 2016
Citation: Berthuy OI, Muldur SK, Rossi F, et al. Organized cell adherent array (OC2A) for real-time multiplex detection of secreted molecules. Int J Biosen Bioelectron. 2016;1(1):13–16. DOI: 10.15406/ijbsbe.2016.01.00004
The organized cell adherent array (OC2A) is an integrated tool useful for real-time and label-free detection of secreted molecules. The OC2A novel cell chip is composed of two substrates, one allowing localized cell culture and the other one constituting the sensitive layer detection. These two units are assembled thanks to a thickness controlled spacer (250μm) which forms a microfluidic lumen. In practice, the top layer of the chip is a polycarbonate substrate covered by a poly (ethylene oxide)-like (PEO-like) film deposited by plasma polymerization. This PEO-like film is cells repellent but allows the direct immobilization of protein through low volume spotting. Thus, fibronectin spots were deposited on this PEO-like film in order to create cell adhesive areas surrounded by non-adhesive background. On the sensitive layer part, various antibodies were immobilized in an organized manner on a surface plasmon resonance imaging (SPRi) chip useful for the multiplex label-free detection of proteins. The two units are then assembled and the cells loaded in the chip. After one hour of culture, non-adherent cells are removed by washing and the chip is ready to experiment. Evidences were obtained of the real-time detection of Prostate Specific Antigen (PSA) and β-2-microglobulin (B2M) secreted by LNCaP cells, androgen-sensitive human prostate adenocarcinoma cells, following induction by dihydrotestosterone (DHT). Different kinetics of the two secreted proteins were also demonstrated and precisely determined using the OC2A system.
Keywords: micro fabrication, cell chip, SPRi, secreted molecules
SPRI, surface plasmon resonance imaging; PSA, prostate specific antigen; B2M, β-2-microglobulin; DMEM, dulbecco’s modified eagle’s medium; PEG, polyethylene glycol; PEO, polythylene oxide; VEGF, vascular endothelial growth factor; PBS, phosphate buffered saline; FCS, fetal calf serum
Recent decades have seen significant progress in the field of biotechnology with the advent of biochips. These new multi parameter analysis tools have allowed considering hitherto unattainable concepts such as sequencing the human genome, but also applications in many fields, especially in the biomedical field.
An understanding of the cellular response to environmental signals is a key issue in the biomedical field including the repair of tissue damage. These stimuli are multiple and can be physical, mechanical, chemical or biological. In addition, the cellular response to these external signals occurs in real time, therefore it is difficult to assess. The need to make these predictable cellular responses and meet the requirements of multiple experiments in parallel has led to the development of cell chips. Indeed, biochips are promising tools to address this type of problem, particularly because of their ability to enable access to tests of parallel and small amounts of biological material required. These cell chips are developed as experimental tools to characterize the relationship between the cell state, environmental stimuli and cellular responses. Biochips have so many important applications such as high-throughput study of target proteins,1 toxic compounds2 and to study the effect of a drug on a cell or a target cell population.3
The production of protein resistant surfaces has always been a subject of great interest and is often considered as a key element in the development of implanted biomedical materials, surface-based diagnostic devices and drug carriers. Polyethylene glycol (PEG) or polythylene oxide (PEO) based hydrophilic surfaces are the most widely used non-adhesive surface due to their robustness and stability. There are numerous techniques used to prepare PEG/PEO based coatings; i.e. chemisorption of thiolated poly (ethylene oxide),4 physical adsorption5 and spin coating stabilized by ion beam treatment.6 These methods, however, are often surface-roughness dependent and usually result in low surface density of PEG/PEO chains with decreased robustness and reduced non-adhesive properties. On the other hand, the use of low pressure plasma polymerization deposition technique of the ethylene oxide precursors n-glyme7 and diethylene glycol dimethyl ether (DIGLYME)8 opened a versatile route for the creation of more robust, dense and homogenous ‘PEO-like’ non-adhesive coatings. Moreover, deposition by plasma polymerization occurs in one step and permits the production of ultrathin coatings (<50 nm) on a large variety of substrates as well as on three-dimensional surfaces. Such PEO-like coatings were also widely used as biological culture platforms for the analysis of cell behavior9 these functional platforms were produced by patterning cell-specific protein arrays (i.e. fibronectin and collagen) directly on an anti-fouling PEO-like background. Such contrasted surfaces allowed the creation of an artificial micro-environment with the ability of controlling the exact position of cells. This can facilitate fundamental studies in cellular research and become essential for the development of cell-based biosensors and biochips.
One of the main reading techniques for biochip is Surface Plasmon Resonance (SPR). Its principle is based on the measurements of refractive index changes at a plane interface between two media with dielectric constants of opposite signs, a dielectric and a metal, such as gold. SPR can be excited when a wedge of polarized light is directed towards the glass face of the sensor surface under the condition of total internal reflection. The resonant angle at which a minimal intensity of reflected light occurs is a function of the local refractive index at or near the gold surface. Such refractive index changes associate intimately with the adsorption or desorption of molecules from the surface, making the technique a powerful tool for bio-recognition measurements.10 To date, all established SPR based sensing platforms have been limited to detection of analyte in a prepared sample.11 In these strategies, sampling of analytes from cell culture media, purification and pre-treatment of analytes are usually required for the purposes of cellular exocytosis and cellular signaling pathways studies.12 These redundant steps are time consuming and also introduce unpredictable errors to the experiments. Therefore, it is desirable to find an alternative method for direct measurement of secretions from living cells. For that purpose. demonstrates a new concept of a SPR biosensor for biomarker study.13 On the basis of integration of a mini cell culture system within a traditional SPR sensing platform, this biosensor was capable of direct measurement of vascular endothelial growth factor (VEGF) biomarker secretion from living ovarian carcinoma cells, SKOV-3. However, this biosensor does not allow multiplex analysis. In the present report we are describing a novel cell-chip design useful for real-time and label-free detection of secreted molecules.
Reagents
Mouse monoclonal Anti-Prostate Specific Antigen (PSA) antibody (ab49395) was purchased from Abcam (United Kingdom). Mouse monoclonal Anti-β-2-microglobulin (B2M) antibody (ABIN641349) was purchased from Raybiotech Inc (United States of America). Anti-CRP antibody was supplied by Meridian Life Science (United States of America). Dulbecco’s Modified Eagle’s Medium (DMEM), Phosphate Buffered Saline (PBS), fetal calf serum (FCS), Fungizone, Penicillin/Streptomycin were purchased from Invitrogen/GibcoBRL (Cergy Pontoise, France). Human fibronectin, 5α-Androstan-17β-ol-3-one (dihydrotestosterone, DHT) and Diethylene Glycol Dimethyl Ether (DEGDME, (CH3 OCH2CH2)2O) were obtained from Sigma-Aldrich (Saint Quentin Fallavier, France).
Poly(ethylene oxide)-like layer (PEO-like) deposition by plasma polymerization
The plasma reactor used in this study was a home-made stainless-steel reactor (vessel size 300 x 300 x 150 mm3) with two symmetrical internal parallel-plate electrodes (diameter of electrodes: 140 mm, distance between two electrodes: 50 mm). The plasma was generated by a radio frequency (RF) generator (13.56 MHz) connected to the upper electrode, whereas the bottom electrode was grounded and used as a sample holder. Plasma polymerization was carried out in continuous plasma discharge mode with a power ranging from 20 to 22 W and a biais of 150 V on a solid support composed of polycarbonate. The reflected power was adjusted to its minimum value (<1% of incident power). Pure Diethylene Glycol Dimethyl Ether (DIGLYME, (CH3 OCH2CH2)2O) vapours (from Sigma Aldrich, used as received) was used as a gas feed for the deposition of poly (ethylene oxide) films (subsequently called PEO-like films) with a monomer initial pressure of around 15 mTorr for a final working pressure of 100 mTorr, a monomer vessel temperature of 45 ± 0.5°C, and a gas line temperature of around 55°C. Gas flow rates were regulated by mass-flow controllers and the pressure was monitored by a baratron (MKS instruments, USA). The low pressure in the chamber was maintained by a rotatory pump. The film obtained is uniform on the polycarbonate substrate with the best antifouling and cell repellency properties at a thickness of 30 nm.14 Between each deposition, the plasma chamber was cleaned for half an hour by a pure oxygen plasma (working pressure at 100 mTorr with a power of 55 W and biais of 400 V). In addition, polycarbonate substrates were also cleaned by oxygen plasma for 5 min before the deposition of poly (ethylene oxide) film. Prior to further experimental stages, samples were carefully washed with ultra-pure water and dried with a nitrogen flush gun to remove possible surplus of monomer and physisorbed material.
Protein microarray
Micro patterning of a protein array on substrates was performed with the S3 sciFLEXARRAYER (Scienion AG, Germany) using the non-contact piezoelectric printing technology. The instrument has a three axis micro-positioning system (accuracy 10 µm) and is equipped with a glass nozzle 80 µm in diameter. The vertical separation between the nozzle and the substrate was typically 0.5 mm. A stroboscopic camera allows visual monitoring to adjust piezo voltages and pulse durations for reliable droplet ejection. Single drops ejected from the nozzle had a volume of 300 pL under our experimental conditions. In our study, 5 drops per spot were dispensed which produced spots of 270 µm diameter size on average. The array of fibronectin was deposited in order to create a mirror image of the antibodies matrix on this PEO film.
For cell adhesive array, fibronectin was diluted to a concentration of 200 µg/ml in phosphate buffered saline (PBS). The spotted sample was incubated for 30 min in a humid environment (70% humidity), followed by exposure to ultraviolet radiation for 5 min and then rinsed in PBS before cell culturing experiment.
For antibodies array, all antibodies were diluted at a final concentration of 200 µg/mL in PBS. In order to deposit a small volume (2.4 nL) of each antibody in an organized manner onto a SPRi chip slide (Genoptics, Horiba, France), a piezoelectric spotter (sciFLEXARRAYER S3, Scienion, Germany) was used. A matrix of 60 antibodies spots with a pitch of 1 mm was created.
Cell preparation
LNCaP cell line (ATCC®- CRL-1740™) was grown on Petri dishes in DMEM supplemented with 10% FCS, 1 mg/mL Fungizone and 50 U/mL Penicillin/Streptomycin at 37°C, in humidity controlled atmosphere containing 5% of CO2. After two days of cell culture, LNCaP cells were passaged by tripsinization and seeded at 2x106 cells/mL.
SPRi experiments
SPRi experiments were performed using a SPRi lab+ (Genoptics, Horiba, France). SPRi slide and SPRi prism were purchased from Horiba (France). All injections in the SPRi microfluidic system were performed with 50 µL/min rate. The fluidic system includes a loop of 500 µL for the injection of the inducer and a space of 100 µL between the two slides. DMEM passes continuously in the system. Experiments were performed at room temperature.
The OC2A novel cell chip is composed of two substrates, the top one allowing localized cell culture and the bottom one constituting the sensing layer detection. These two units are assembled thanks to a thickness controlled spacer (250µm) which forms a microfluidic lumen. A schematic representation of the cell-chip configuration is presented in (Figure 1). In practice, the top layer of the chip is a polycarbonate substrate (25 x 12 x1 mm) coated with a PEO-like film deposited by plasma polymerization. This PEO-like film is non-adhesive for cells but allows the direct bio-patterning of protein.15 Fibronectin spots are then deposited on this PEO-like film in order to create cell adhesive areas surrounded by the non-adhesive background. On the bottom layer, various antibodies are immobilized in an organized manner on a SPRi chip slide and permitted the multiplex detection of different proteins secreted by the cultured cells. The fibronectin spots matrix is a mirror image of the antibodies spots matrix, thus permits cells to be immobilized in front of each antibody. After assembly, a cell suspension is load and seed into the chip which is turn over in order to have the cell culture surface at the bottom and then cultured for 1h (Figure 2A).
The micro patterned PEO-like film is an efficient substrate for the localized and controlled cell culture. Indeed, after only 1h of culture and gentle wash with PBS, adherent cells are found only on top of the fibronectin spots (Figure 2B). In order to demonstrate the possibility to detect in real time and label free the kinetic of the molecules secreted by cells on the chip, we have been working on a model cell line, the human prostatic carcinoma adherent cell line LNCaP. This cell line has the particularity to secrete Prostate Specific Antigen (PSA) and β-2-microglobulin (B2M) in response to a specific chemical stimuli, the presence of dihydrotestosterone. LNCaP cells have been seeded at 2x106 cells/mL for one hour in order to have about 25 cells per spot for SPRi experiments. In order to demonstrate direct measurement of B2M and PSA from living prostatic carcinoma cells, the chip was assembled with a SPRi prism and load into the SPRi apparatus. Four different parameters were followed using the SPR signal (Reflectivity), the SPR drift in front of adherent cells on gold, the SPR variation on anti-PSA antibodies immobilized in front of adherent cell, the SPR variation on anti-B2M antibodies in front of adherent cells and the SPR variation on non-specific antibodies (anti-CRP) in front of adherent cells. The results are summarized in (Figure 2C). As can be seen, both B2M and PSA were detected in the first 30 minutes following the DHT induction. This is enough to recorder quantitative detection of analytes but the data was recorded when we observed a saturation effect. In order to evaluate the quantity of protein secreted by cells on each spot, reflectivity variations were converted to amount of molecules per unit area (in pg / mm²) using the following equation (equation given by Genoptix):
τ= "∆RLZC" /(S"P,R " δn/δc)
Where ΔR is the reflectivity variation in percentage, LZC = 1.02x10-4 mm (depth of penetration of the plasmon wave), SP, R = 2.25x103 %/RIU (sensitivity of the SPR as a percentage per unit of refractive index) and Δn/δc = 1.9x10-10 mm3/pg. The highest reflectivity variations are 1.3% and 0.8% for PSA and B2M, respectively, corresponding to 310 pg/mm² of PSA and 191 pg/mm² of B2M. The antibody spots have an average diameter of 250 microns which corresponds to a surface of 49062.5 μm². It is then possible to estimate the amount of protein per spots: 15 pg for PSA and 9 pg for B2M. There is about 30 adherent cells per antibodies spot. So one cell would have secreted 0.5 pg pf PSA and 0.3 pg of B2M 1h 20 after inductions. Such an early detection of the secreted PSA and B2M in our micro system was attributed to the overconcentration effect of the secreted proteins, possible only thanks to the 250 µm thick lumen of the OC2A in which secreting cells are in close environment with the detection layer.
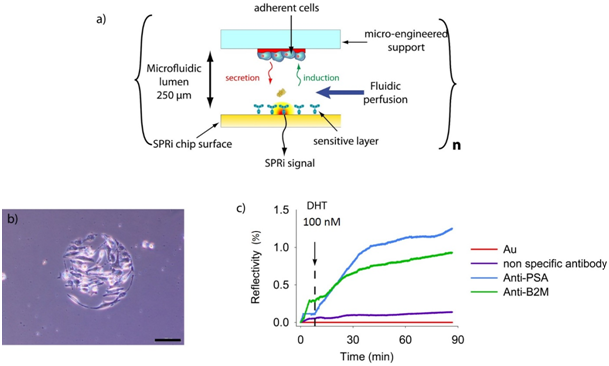
a) Schematic representation of the OC2A concept for real-time detection of secreted molecules by small populations of adherent cells.
b) LNCaP cells adherent on a fibronectin spot after 1h of culture observed with bright field microscopy using a 10x objective (Olympus) (the scale bar represents 100 µm).
c) Real-time detection of PSA and B2M secreted by LNCaP cells after DHT 100 nM induction.
We were able to successfully design a “closed” biochip, easy to perform and has the advantage of being made in advance, stored and transported (4°C) before the injection of cells and does not require special equipment. In addition, with this concept, the time of adhesion of cells is very short (1 hour against 4 hours in standard cell culture conditions). Evidence of the real-time detection has been presented for PSA and B2M secreted by prostate cancer cells (LNCaP) following induction by DHT using the OC2A system. In addition, the response of cells to the inductor could be observed after only 30 minutes). However, the signal strength is lower because of diffusional constraints but is very specific. There is no doubt that the OC2A system will, in the near future, be applied to more multiplexed and complex biological secretion systems for which kinetic data are not reachable at the moment using standard cellular biology tools.
None.
The author declares no conflict of interest.

©2016 Berthuy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.