International Journal of
eISSN: 2573-2838


Research Article Volume 3 Issue 2
1Department of Exact and Earth Sciences-Laboratory of Physics (Micro fluidic sector), State University of Bahia, Brazil
2CPGEI, Federal Technological University of Paran (UTFPR), Brazil
Correspondence: Walter Duarte De Araujo Filho, Department of Exact and Earth Sciences-Laboratory of Physics (Micro fluidic sector), State University of Bahia, Street Silveira Martins, 2255, Zip zone: 40150 000, Salvador-Bahia, Brazil, Tel +5571992645841
Received: September 01, 2017 | Published: September 20, 2017
Citation: Filho WDA, Araújo LMP. Monodisperse microbubbles as drug carrier units having the olive oil as the coating layer from devices manufactured by 3d printing. Int J Biosen Bioelectron. 2017;3(2):252–255. DOI: 10.15406/ijbsbe.2017.03.00059
The use of microbubbles as drug delivery units has attracted much interest since it has been envisaged that a biologically active compound may be brought to localized regions of the body for the treatment of various types of diseases, such as cancer. The microbubbles are brought to the chosen site through the bloodstream, monitored by a transducer which also blows them using ultrasonic pulses, thereby releasing the drug or active principle at the desired location. Such a procedure avoids or minimizes the side effects resulting from conventional treatment, providing a better quality of life to the patient during treatment. This work deals with the generation and monodisperse microbubbles, that is, with a high degree of homogeneity in relation to the physical and morphological characteristics: shape and size. The device was able to generate microbubbles with a mean diameter of 18.76μm with a rate of variation of 1.03 % which characterizes a monodisperse population. The microbubbles generated have the coating layer of lipid nature consisting of olive oil and an associated surfactant, Polysorbate-80, which, due to its amphiphilic properties, forms the bilayer structure of the microbubble. The next step is to attach biologically active compounds extracted from plants from the Brazilian northeastern semi-arid to the microbubbles and initially test in vitro the behavior of tumor cells after the release of the drug through ultrasonic pulses.
Keywords: microbubbles, ultrasound, 3D printing
The use of micro bubbles as auxiliary units in the diagnosis of diseases began in the 1980s, when they were used to increase the contrast of ultrasound images in echocardiographic examinations.1-3 Microbubbles are small microspheres loaded with a specific gas that have specific acoustic properties, which make them very useful as ultra-sonographic contrast agents for diagnostic imaging (Figure 1). Micro bubbles as carriers of pharmacologically active compounds represent one of the most promising frontiers of modern medicine having great potential to revolutionize disease treatment, specifically where the high concentration of drugs administered systematically causes undesirable side effects to the patient. Currently, many researches on this subject are underway, seeking to improve micro bubble production techniques by looking for new biocompatible coating matrices4-10 and at the same time satisfies the prerequisites of stability and ability to withstand the aggressions of the environment in which they will be dispersed. The feasibility of this new modality of treatment involves the development of techniques of manufacture of generators, accessible and, at the same time, able to generate uniform and stable micro bubbles with sizes compatible with clinical applications, i.e. with diameters of the order of 10 µm (approximate size of a red blood cell).

Recent developments have demonstrated the feasibility of using micro bubbles as transporters for local administration of drugs suitable for the treatment of tumors,11-14 Well-known for its use in real-time imaging without dangerous irradiation for living tissues, ultrasonography can also be used to control the release time of the drug when carried by a micro bubble. This type of therapeutic application is a promising treatment modality, particularly in cases where high concentrations of drugs are administered systematically, causing significant side effects to the patient.15-17 Overcoming these problems related to drug administration leads to a better quality of life for patients, reducing the possibility of secondary hospitalization during treatment. The proposed work proposes the generation of monodisperse micro bubbles of lipid coating layer with olive oil as lipid matrix. In order to arrive at the proposed results, microfluidic devices were manufactured using the 3D printing technique. This technique of manufacturing of the generating devices proved to be quite efficient, besides producing significant results related to the uniformity and low dispersion of the micro bubble population produced.
Generation of micro bubbles
The micro fluidic system used to produce the micro bubbles was T-junction (Figure 2). The channels forming the upper part of the trucking arm are connected to a cylinder which supplies the gas at a given flow rate and constant pressure. The channel forming the underside of the arm is connected to a syringe pump, which controls the rate of flow of the liquid phase. A bottleneck process due to instability at the gas liquid interface forms the microbubbles.18 The microfluidic device design was developed in the Solid Works environment with the SLDPRT extension, and manufactured from a 3D OBJET EDEN 250 printer using the Vero Clear RGD810 transparent resin. Figure 3 shows the fabricated device used to generate micro bubbles. Figure 4 shows in detail the geometry of the according to internal dimensions. The minimum diameter of the device channels is 300 mm. This value represents the maximum resolution of the equipment in the manufacture of closed channels, which is a limitation when it is necessary to work with channels of smaller dimensions. To circumvent this limitation, a micropipette of maximum outer diameter (body) of 1.0 mm was encapsulated to the device (Figure 4). The micropipette coupled to the device has the function of inflating the microbubble. The opening of the gas phase in the device is directly connected to the size of the microbubble generated as shown by the experimental equation presented by 19,20 i.e.:
Where D is the diameter of the microbubble, QG represents the gas phase flow rate, QL the liquid phase flow rate, α a proportionality constant and Wd represents the opening of the gas phase feed channel. The proportionality constant for this equation depends on the geometric characteristics of the device such as the profile of the channels (circular profile), but is almost independent of the properties of the fluid. In the specific case, α assumes the first order value (i.e., α = 1).20

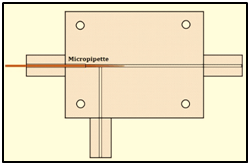
Thus, a micropipette whose tip had an internal diameter of 18 μm corresponding to the gas phase aperture (Wd = 18 μm) was coupled. The operation of the microfluidic device is done in biphasic regime as shown in Figure 5. The gas phase feed is performed through a gas cylinder containing nitrogen (N) of molar mass of 14.01 g/mol and viscosity of 0.0177 cPa The pressure and flow rate are controlled by the devices SMC AS2000 and SMC AS2001. The liquid phase is fed through a hospital syringe pump ST670-SAMTRONIC INFUSION SYSTEMS. The generation of the microbubbles was monitored through a high-speed camera VIS SDK 7.2.1 coupled to a stereomicroscope XTL series - STEREO ZOOM MICROSCOPE. The liquid phase consisted of an emulsion with water, olive oil and Polysorbate - 80 (Tween 80) having the 98: 1.5: 0.5 mass ratio. All generation process took place under a temperature of 22oC and Salvador-Ba atmospheric pressure of 1021hPa. Figure 5 shows the experimental apparatus used to generate microbubbles. The captured images were automatically processed through a computational tool in the MATLAB® environment, as well as the statistical survey of the microbubble population.
Figure 6 shows the passage of the microbubbles of the device channel micro fluidic T-junction manufactured according to the 3D printing technique. The experimental results demonstrated the efficiency of the device in achieving the proposed objectives. Table 1 shows the diameters, radius, perimeters and areas of 28 microbubbles generated in time of 1.0 s. Figure 7 shows the normal distribution of the microbubble diameter associated with the histogram for a gas phase flow QG = 7.74×10-4 µl/s, liquid phase flow and QL= 1.38 ml / s and relative viscosity of liquid phase of ηr = 1.23 mPa.s. Twenty-eight microbubbles were generated in the time of 1.0 s with a mean diameter of 18.76 mm, standard deviation of 0.19 m and coefficient of variation (Vc) of 1.03% (Table 2).
Microbubbles |
Radius [mm] |
Diameter [mm] |
Perimeter [mm] |
Area [mm] |
1 |
9,38 |
18,75 |
58.9 |
276,11 |
2 |
9.48 |
18,95 |
59.53 |
282,03 |
3 |
9.44 |
18,88 |
59.31 |
279,95 |
4 |
9,52 |
19,03 |
59,78 |
284,42 |
5 |
9,11 |
18,21 |
57.21 |
260,43 |
6 |
9,55 |
19,09 |
59.97 |
286,21 |
7 |
9.44 |
18,87 |
59.28 |
279,65 |
8 |
9,52 |
19,03 |
59,78 |
284,42 |
9 |
9,31 |
18,62 |
58,50 |
272,29 |
10 |
9.32 |
18,64 |
58.56 |
272,88 |
11 |
9,38 |
18,76 |
58.94 |
276,40 |
12 |
9,37 |
18,73 |
58,84 |
275,52 |
13 |
9,51 |
19,01 |
59.72 |
283,82 |
14 |
9,29 |
18,57 |
58,34 |
270,83 |
15 |
9.34 |
18,67 |
58,65 |
273,76 |
16 |
9,35 |
18,69 |
58.72 |
274,34 |
17 |
9,34 |
18,67 |
58.65 |
273,76 |
18 |
9,37 |
18,74 |
58,87 |
275,81 |
19 |
9,36 |
18,72 |
58,81 |
275,23 |
20 |
9,51 |
19,01 |
59.72 |
283,82 |
21 |
9,33 |
18,66 |
58,62 |
273,46 |
22 |
9,51 |
19,02 |
59.75 |
284,12 |
23 |
9,36 |
18,71 |
58,78 |
274,93 |
24 |
9,33 |
18,65 |
58.59 |
273,17 |
25 |
9,27 |
18,54 |
58.25 |
269,96 |
26 |
9,31 |
18,62 |
58,50 |
272,29 |
27 |
9,34 |
18,67 |
58.65 |
273,76 |
28 |
9,39 |
18,78 |
59,00 |
276,99 |
Table 1 Diameters, radius, perimeters and areas of 28 microbubbles generated in time of 1.0 s
Minimum Diameter ip.mi |
18.21 |
Maximum Diameter [um] |
19.09 |
Mean Dianieter [pini] |
18.76 |
Standard Deviation [pm] |
0.19 |
Variation Coefficient Vol |
1.03 |
Table 2 Statistical data regarding the 28 microbubbles generated in time of 1.0 s

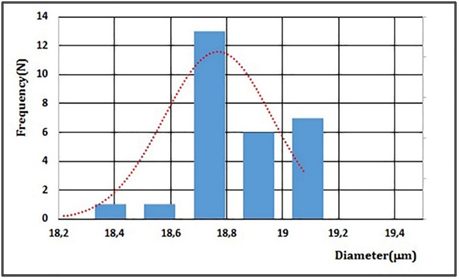
The results presented show the capacity of the system proposed to generate microbubbles with mean diameter (Dm) of 18.76 mm and coefficient of variation (Vc) of 1.03% (Table 2), which characterizes the monodisperse character of the microbubble population produced.18 This characteristic is very important because it is associated to the increase of the therapeutic action, since the frequency of the ultrasonic field has a near direct relation to the diameter of the microbubble and the homogeneous character of the population, considering that a narrow range of frequency potentiates the cavitation of the population of microbubbles and the consequent destruction of them, providing a greater release of the drug at the chosen site.18 The next step of the work consists of adding a biologically active compound from plants from the Brazilian Northeast semiarid to the microbubbles and studying the dynamic behavior of the generation process, besides the study of the stability of the microbubble population from the use of new lipid matrices. It should be noted that for clinical applications it is necessary that the mean diameter of the microbubbles be of the order of 10 μm, which has not yet been achieved in this work. More accurate and precise control of the gas phase associated with the micropipette encapsulation with orifice diameters of the order of 10 mm can solve this problem, approaching even more the proposed objectives.
Based on the results obtained, it can be concluded that the proposed device was able to produce monodisperse microbubbles with low coefficient of variation (1.03%), which makes possible its application for the generation of microbubbles for clinical use. The use of olive oil as a lipid matrix played an important role in the generation process, making it possible to achieve the aforementioned results. Future works related to the reduction of microbubbles diameter, tests with new lipid matrices besides the annexation of bioactive components extracted from plants would be implemented in order to enable the clinical use of microbubbles.
Thanks to the State University of Bahia (UNEB), and the Federal Technological University of Paraná (UTFPR) for providing the necessary conditions for the development of this work.
We declare no financial interest or any conflict of interest.

©2017 Filho, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.