International Journal of
eISSN: 2573-2838


Mini Review Volume 4 Issue 3
Ruder Boskovic Institute, Croatia
Correspondence: Ciglenecki I, Division for Marine and Environmental Research, Ruđer Boskovic Institute, Bijenicka 54, 10000 Zagreb, Croatia
Received: April 19, 2018 | Published: May 4, 2018
Citation: Ciglenecki I, Marguš M, Orlovic-Leko P. Mercury electrode as a tool/sensor for pollutants monitoring in natural waters; advantages and disadvantages regarding moving to “green” electrochemistry.Int J Biosen Bioelectron. 2018;4(3):94–96. DOI: 10.15406/ijbsbe.2018.04.00104
Electrochemical measurements are the most used and challenging approaches in trace elements analysis and speciation in complex natural samples. There is a wide research community that used electrochemical methods for studying critical environmental and biogeochemical processes, both natural and anthropogenically perturbed, that influence ecosystem services and human health. In recent years more attention is focused on development of environmentally friendly and sustainable chemical processes and tools which have aim to reduce negative footprint on the environment. Development in the electrochemistry is heading in this “green” direction, since EU regulations confined use of mercury, which has been established as the preferred tool/sensor in electrochemical measurements. Therefore, development of new and more environmentally safely electrode materials is imperative. In this mini-review we discuss advantages of the mercury electrode use in measurements of organic material with surface active properties and sulfur compounds in natural waters.
Keywords: electrochemistry, voltammetry, Hg electrode, pollutants, chemical speciation, natural waters, green electrochemistry
Green Chemistry or Sustainable Chemistry is defined by the EPA1 as "the design of chemical products that reduce or eliminate the use of hazardous substances". In recent years there are great expectations that chemists will produce greener and more sustainable chemical processes. In this direction, relevant benefits from “green” electrochemical activities (better control, higher selectivity, safer operations, milder conditions, and the use of electrons as a cheap reagent) were found. Some important instances of “green” electrochemistry are detectors and bio-detectors for environmental analysis (detection of pollutants). Since the invention of polarography, the Hg electrode was established as the preferred electrode for use in electrochemistry due to the high over potential for hydrogen evolution (which enables work at moderately negative potentials), as well as renewability of the electrode surface.2 The current trend of “green” chemistry, that encourages avoiding the use and generation of toxic substances, as well as recent EU regulations that have prohibited exporting and storing metallic Hg, have led to the search of competitive electrodes fabricated with minimal amount of Hg or definitely with other materials. However, in comparison to solid electrodes, Hg has many advantages which cannot easily be ignored and replaced in the move towards “green” electrochemistry by use of solid electrodes when characterizing the surface active (SA) fraction of organic matter as well as sulfur species in natural waters.2–6
There is a wide range of electroanalytical techniques which use the mercury electrode as a working electrode for possible qualitative and quantitative determination of pollutants in natural waters (potentiometry, polarography, voltammetry, chronopotentiometry, chronoamperometry etc.).7,8 These electrochemical methods, especially voltammetry, have appropriate features to be used as monitoring methods (early warning tools) for aqueous systems in general and are among key methods for analyses of hydrophobic and surface active organic compounds2-4, sulphur species,5,10–16 trace metals,8,17–22 engineered and natural nanoparticles.13–16,23–25. Voltammetry is the only technique allowing determination and chemical speciation (oxido-reduction state, the presence of different species) of the truly dissolved and particulate inorganic and organic species without any sample handling. Given that voltammetry provides information about relationship and reactivity between different chemical species in natural waters. So we can say that it allows monitoring the interaction between chemistry, biology, physics, and/or it provides answer how chemistry affects biology and ecology, and/or how living organisms change the chemistry of the environment where they live.6,9,21,26 Thus, electrochemical measurements in natural waters are essential in order to obtain more complete speciation information and to fully understand the geochemical cycling and bioavailability (toxicity) of elements. To fulfill this mission it is mandatory to have enough sensitive working electrodes, i.e. voltammetric sensors, with embodiments that make them specific for detection of natural and anthropogenically introduced compounds in natural environment.
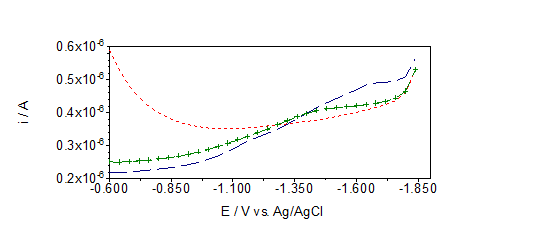
Electrochemical methods that have been applied in our group in the last 50 years enables quantification of different type of organic matterial with surface active properties, organic and inorganic sulfur compounds (reduced sulfur species,RSS), natural and engeenered nanoparticles in model and different natural water samples (urban and marine atmosphere, sea- and freshwater systems, drainage water from agriculture area, mining water). Developed methodology offers insight into spatial distribution and seasonal variations of surface active substances (SAS) 3,4,27–29 and RSS6,9,10,13,27 in the water environment. Curve shape analysis (Figure 1) enables rough characterization of predominant SAS groups (more hydrophobic and/or hydrophilic types, from saturated fatty acids, polysaccharide xanthan, fulvic and humic type of material), 6,4,27,29 while oxido-reduction peaks offer information on presence of analytes that are oxydized and/or reduced at the Hg surface,4,5,12-16 (Figure 2). A detailed analysis of results from both approaches gives information about the composition of the investigated samples, and prevailing type of organic material.3,4,27,29
Hg electrode is a simple and very practical model of a charged interface while solid electrodes resemble natural interfaces more closely, especialy for the study of adsorption processes and surface active substances measurements.30,31 In comparisson with Hg, Au electrode has stabile structure which is more sensitive on the way of electrode preparation, applied potentials, composition of the solution, and temperature.29,30 Comparative measurements of sulfide at the Hg and Au electrodes showed different sensitivity and selectivity of the Hg surface for such type of compounds. Besides, advantages of smooth and reproducible renewable surface of the Hg electrode have been evidenced in analytical procedures based on both faradaic and nonfaradaic processes. Nevertheless, in our measurements and water quality monitoring we have not yet found an adequate and more „green“ replacement for the Hg electrode.
Desorption peak around -1.6 V in the NF sample can be ascribed to the presence of polysacharide type of organic matter3. Please note that majority of this material was removed by filtration through 0.45 um filter. Dotted red curve corresponds to electrolyte (0.55 M NaCl). All curves are recorded after 120 s accumulation time (with stirring of the solution) at the deposition potential of −0.6 V at the hanging Hg drop electrode. All potentials were measured against the Ag/AgCl reference electrode while graphite rod was the counter electrode. Cathodic going scan without accumulation (dotted line) and after 120s of accumulation (dash line) at Eacc= 0 V. Scan rate=100 mV/s. Reduction peak at around -0.6 V corresponds to the presence of sulfur species recovered by HgS reduction,6,10 while anodic peaks at around -0.8 and -0.4 V correspond to the presence of Fe.14–16 Please note that all peaks were recovered by longer accumulation time at the starting potential. All potentials were measured against the Ag/AgCl reference electrode; graphite rod was the counter electrode.
This work is supported by the project "The Sulphur and Carbon Dynamics in the Sea and Fresh-Water environment" (IP-11-2013-1205 SPERE).
Authors declares that there is no conflict of interest.

©2018 Ciglenecki, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.