International Journal of
eISSN: 2573-2838


Review Article Volume 2 Issue 4
Mechanical Engineering Department, San Jose State University, USA
Correspondence: Winncy Y DU, Mechanical Engineering Department, San Jose State University, San Jose, California, 95116, USA, Tel +1-408-924-3866
Received: April 22, 2017 | Published: May 2, 2017
Citation: Du WY, Jose W. Design of an ECG sensor circuitry for cardiovascular disease diagnosis. Int J Biosen Bioelectron. 2017;2(4):120–125. DOI: 10.15406/ijbsbe.2017.02.00032
ECG biosignal conditioning (BC) is critical because it directly affects measurement accuracy, reliability, and repeatability. It also presents a great challenge due to the small amplitude of ECG raw signals and their ease of corruption with noise and other disturbances. This paper describes how an ECG biosignal conditioning circuitry was designed, built, and tested. The circuit consists of an instrument amplifier (AD8220), a 1st-order active high-pass filter (containing a MCP6271 op-amp and a RC filter in Sallen-Key configuration), a 5th-order active Bessel low-pass filter (consisting of a 1st-order LPF and two 2nd-order Sallen-Key filters), and a Twin-T active notch filter (combining two “T” shape RC filters with a MCP6271 op-amp). A right leg drive circuit was added in order to cancel the common-mode signal between the left and right arm electrodes. A power supply circuit provides a ±5V DC source for the system using two 9V batteries and two voltage regulators (NTE977 regulates +9V power supply to +5V; NTE1917 regulates -9V supply to -5V). Data acquisition and sampling were performed using a USB6009 module with a built-in A/D converter. Testing of a real electrocardiogram from a human subject was performed on the designed BC circuit, which has indicated that the developed BC circuitry can preserve useful ECG information while removing unwanted noise and interference components.
Keywords: biosignal conditioning, ECG, passive and active filters
Sensors have been playing key roles in all aspects of health care, especially Cardiovascular disease (CVD) -the disease that is the No.1 killer in the United States and the single leading disease that ended 17.3 million people’s lives worldwide in 2013.1 Unlike many other diseases, some CVDs can cause sudden death, especially in people who physically appear heathy and fit. Early diagnosis and warning, therefore, are extremely important to uncover hidden or silent heart diseases and prevent sudden death. In addition, the cost for detecting and treating cardiovascular disease is very high, thus early diagnosis and prevention post a significant financial benefits as well. Sensing and monitoring technologies for heart deceases have attracted great attention from health care, engineering, industry and information processing societies.
The sensor technology and development for CVD studies and diagnosis mainly focus on
The electrocardiogram (EKG or ECG) is a graph that records the electrical activity of the heart. Its amplitude has a range of 0.1 mV-10 mV; while its frequency falls into 0.01 Hz-250 Hz. ECG is an important tool used for support of diagnosis and treatment of various cardiac and other related diseases:
Be the first indicator of ischemia and help in proper use of thrombolysis in treating ischemia.
An ECG is usually obtained by means of metal electrodes placed on the surface of the body. However, the raw ECG signals directly from the electrodes are inherently weak (0.001 mV-100 mV with a typical value of 1 mV. They are easily corrupted with noise and other disturbances such as power line interference, impulse noise, electrostatic potentials, stray capacitance, and nearby electronic devices. Biosignal artifacts can also be introduced into an ECG by subject movement and muscle tension.2 All of these facts have posted a great challenge on ECG data. The biosignal conditioning (BC), therefore, has played a key role in ECG and many other biomedical instruments and biosensors. A well designed BC circuit can significantly improve measurement accuracy, reliability, and repeatability.
Several works on ECG sensing have been reported in the literature.3-12 Tenedero et al.3 developed an ECG circuit with a bandwidth of 0.05 Hz - 40 Hz. An AD620 instrumentation amplifier (IA) was employed due to its low noise, low input bias current, low offset voltage, low power, and high Common Mode Rejection Ratio (CMRR) of 100 dB.3 Three filters were used between the IA and the data acquisition unit: an isolation amplifier (isolates 60 Hz power line and also protects the patient from cardiac shock), a high-pass filter with a cutoff frequency of 0.05 Hz, and a low-pass filter with a cutoff frequency of nearly 100 Hz. The sampling rate in the ADC (analog to digital conversion) for the ECG signal is 500 Hz. Fulford-Jones et al.4 designed a portable, low-power ECG system.4 An operational amplifier (op-amp) embedded in a single INA321 chip was used due to its low noise and low power consumption. This op-am has a CMRR of 94 dB. A high-pass feedback filter corrects any DC shift occurring over time. Their ADC sampling rate is 120 Hz. Matviyenko5 used a CY8C27443 microcontroller for ECG signal acquisition and processing. The IA embedded in the controller has a CMRR of 60 dB. According to the author, this low CMRR was acceptable due to the unique design which placed a differential low-pass filter before the IA to reduce radio frequency interference (RFI), because RFI error cannot be filtered out after the ECG signal has been rectified by the IA. A 2 kHz cutoff frequency high-pass filter was placed at the output of the IA. A buffer amplifier and an inverting amplifier were also used to cancel the RFI interference. The ADC sampling rate is 240 Hz. Two industry leaders, Texas Instruments (TI)6 and Analog Devices Inc. (AD),7 also developed the ECG conditioning circuits. TI’s circuitry features an INA321 IA with several unique features: a power down mode that shuts down the circuit when the supplied current is less than 1 mA (for power saving), embedded op-amps in the microcontroller, a feedback loop that maintains a constant DC level, and a 512 Hz sampling rate. Further filtering was digitally implemented to remove power line noise and to provide a pass band of 6 Hz-30 Hz. In AD’s design, the ECG circuit uses AD’s AduC842 (an integrated “system on chip”) to perform amplification, digital filtering, and A/D conversion. This paper discusses the design of an electrocardiogram (ECG) sensor circuitry for cardiovascular disease diagnosis. The paper starts with obtaining the real ECG signals (Section II), followed by signal conditioning circuit design and implementation (Section III). ECG Signal testing and results using the designed BC circuit are described and shown in Section IV. Finally Section V draws the conclusions.
Obtaining an real ECG signal
An Electrocardiogram (ECG) is usually recorded by electrodes placed at specific points on the body. The electrode used for recording the ECG signal is silver-silver chloride (Ag-AgCl). It has the following important features: (1) it is non-polarizable, meaning that current flows freely across the electrode junction. No electrons accumulate in the junction as in a polarizable electrode; (2) it generates less noise (<10 µV). The Ag-AgCl electrode has an AgCl layer deposited on an Ag plate. The Cl- ions move in the human body (in the electrolyte). In the AgCl layer, these Cl- ions are converted to electron flow in the Ag plate and these electrons are then sent out through a connecting wire. This Ag-AgCl structure reduces the DC offset potential to a very small value. A conductive gel is used to minimize the disturbance of the double charge layer. Three limb leads are commonly used to construct an Einthoven’s triangle (Figure 1).13
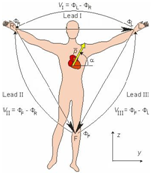
Figure 1 Einthoven’s triangle and limb lead configuration.13
An ECG wave can be recorded by placing leads at electrically equidistant points on the body from the heart, thus maximizing the potential difference between leads.14 Lead I is configured as the positive electrode on the left arm (L), the negative electrode on the right arm (R); Lead II is configured as the positive electrode on the left leg/foot (F), the negative electrode on the right arm (R); Lead III sets the positive electrode on the left leg/foot (F), the negative electrode on the left arm (L). VI, VII, and VIII denote the voltage of Lead I, Lead II, and Lead III, respectively. ∅L, ∅R, and ∅F are the voltage potentials at the points L, R, and F, respectively. The Cardiac Vector’s magnitude and direction a can be expressed by:14
or
or
The typical ECG signal obtained from the Ag-AgCl electrodes is 1 mV in amplitude and it is easily corrupted by noise. The main sources of noise include respiration, motion artifacts, muscle contraction, electrode contact noise, power line interference, RFI, and electromagnetic interference (EMI). In certain situations, noise can completely override the ECG waves and make the amplified signal useless.
Signal conditioning circuit design and implementation
To effectively remove unwanted noise and preserve the useful components of ECG signals, the following biosignal conditioning schemes and sequence were developed:
A block diagram of the BC circuit is shown in Figure 2. The following sections will discuss component functions and features.
AD8220 instrumentation amplifier
An Analog Devices’AD8220 instrumentation amplifier (IA) was used first to amplify the ECG signal obtained from the potential difference between the right and left arm electrodes (Lead I in Figure 1). The AD8220 has a wide operating range in noisy environments, low input bias current of 10 pA, high CMRR with little effect from RFI, and adjustable gain.15 Since the voltage source Vs of the AD8220 is ±5V, the gain G was conservatively set at 19 to avoid both output voltage saturation, although a higher gain is achievable with this IA. At this setting, a typical 1.0 mV ECG signal is amplified to 19.0 mV -well below the Vs level. The gain resistor Rg is calculated by:16
First-order active High-pass Filter (HPF)
After the amplification, the ECG signal passes through a non-inverting active high-pass filter (HPF) to eliminate DC offset (developed between the electrodes) and to be amplified further. This active HPF consists of a MCP6271 op-amp, a RC filter (capacitor Z2 and resistor Z4) and the gain resistors Z10 and Z11 arranged in a Sallen-Key configuration (Figure 3). The bandwidth of the ECG recording system is very important in order to achieve an accurate recording of cardiac signals. The American Heart Association (AHA) recommends a minimum bandwidth of 150 Hz for children between the ages of 12 to 16 years; and a minimum bandwidth of 125 Hz for adults.17
Based on these recommendations, the ECG signal was set to a frequency range (bandwidth) of 0.01-100 Hz. To ensure the most useful ECG signals pass through the HPF, a cutoff frequency fc of 0.033 Hz, instead of 0.01 Hz, was chosen in consideration of manufacturing tolerances for electronic components. Z2 was set to 6.8 mF for a faster response. Thus, the resistor Z4 has a value of:
In addition, Z10 and Z11 were set to 806Ω and 13kΩ respectively. The resulting HPF gain, GHPF, is:
Fifth-order active bessel low-pass filter
After the HPF, the ECG signal is sent to a low-pass filter (LPF) to remove its high frequency noise components. 160 Hz was chosen as the cutoff frequency based on the normal ECG frequency range as well as the AHA guidelines for minimum ECG frequency bandwidth. The LPF is a cascaded 5th-order active Bessel filter which has excellent transient and linear phase response.18 In comparison, other filter types, like the Chebychev, have overshoot (ripple) in their passband magnitude response and transient response. An active filter kit from Microchip Technology was employed for the filter setup. A total of three active filters were needed to form this 5th-order Bessel filter (Figure 4).
The first filter is an active 1st-order LPF which has a real pole in its transfer function; the second and the third filters both have 2nd-order Sallen-Key topologies (each has a pair of complex conjugate poles in their transfer function). All three filters use unity gain (the Sallen-Key topology inherently has excellent gain accuracy as a unity-gain buffer). The unity gain configuration also requires less components-two resistors versus four resistors in non unity-gain designs, thus reducing thermal noise caused by resistors. The resistors and capacitors in the LPF were determined as follows, considering Filter 2 first:
If m = 1, then from (10) n = 4.
C should not be too small since low capacitor values can result in significant errors due to parasitic capacitance. Chose C = 0.068 µF, thus:
From equation (8),
Similarly, Filter 1 can be designed by letting fc = 160 Hz, Q = 1, n = 4.5, m = 2, C = 0.068 µF, and calculating C11 = 0.068 µF, R11 = 14.628 kΩ; Filter 3 designed as C31 = 0.306 µF, C32 = 0.068 µF, R31 = 9.752 kΩ, and R32 = 4.876 kΩ. All op-amps in the LPF are MCP6271. This CMOS chip has a 2 MHz Gain Bandwidth Product (GBWP) and a 65° phase margin. It also supports rail-to-rail input and output swing.19
Notch/Band-reject filter
A notch or band-reject filter is typically used in biomedical instrumentation to suppress a certain frequency or range of frequencies in signals. The 60 Hz power field interference needs to be rejected. This requires a small transition bandwidth or high Q factor to achieve the steeper roll-off. A Twin-T notch filter is one of the few RC networks capable of providing an infinite deep notch at a particular frequency. Two “T” shape RC filters combined with a MCP6271 op-amp, as shown in Figure 5a, form an active notch filter. The Q factor can be raised from the usual 0.3 to 2.5 (a Q factor of 50 or more is achievable). Further, the op-amp provides low output impedance and high input impedance, making it possible to use large resistance values in the “T”' so that only small capacitors are required, even at low frequencies.
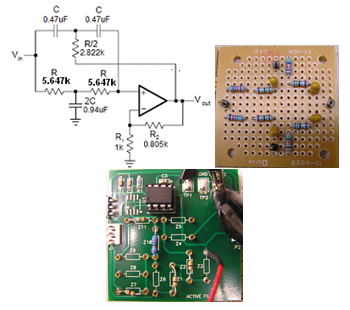
Figure 5 The Twin-T notch filter to remove 60 Hz powerline noise (a) Notch filter circuit board: passive portion (b) and the active portion (c).
With fn = 60 Hz and C = 0.47 µF, the resistor value in the filter is:
Another advantage of the Twin-T configuration is that the quality factor, Q, can be altered via the inner gain G without modifying the notch frequency fn. To achieve a Q factor of approximately 2.5, resistances of R1 = 1.0 kΩ and R2 = 805 Ω were chosen,
The resulting filter gain is:
The designed notch filter is shown in Figure 5b & 5c shows the passive portion of the Twin-T filter, while Figure 5c shows the active portion of the filter. The resistors placed in series on the passive portion provide a total resistance of 5.647 kΩ; while the two resistors, R1 and R2, on the active filter board set the Q factor and gain G of the filter. A +5V power supply is provided to the MCP6271 (similar to Vdd in an LPF circuit); all grounds on both the passive and active boards are connected to a 2.5 V reference (similar to Vdd/2 in an LPF circuit).
Right leg drive circuit
A right leg drive circuit is used to cancel the common-mode signal between the left and right arm electrodes by inverting, amplifying, and then feeding the signal back to the body through the right leg electrode. As shown in Figure 6, there are three leads connected to the body: a left arm lead, a right arm lead, and a right leg lead. Two AD708 dual op-amps are used: the first one acts as a buffer with a unity gain, and the second one acts as an inverter and amplifier (i.e., inverting amplifier). A gain of 68.19 was achieved with the 866 kΩ and 12.7 kΩ gain resistors (i.e., G = 866/12.7 = 68.19). A 0.068 mF capacitor in the feedback loop was used to maintain the stability of the right leg drive circuit. The 499 kΩ resistor at the output end limits the current driven to the body for patient protection. This right leg drive circuit was built on a 3.81 cm x 3.81 cm prototyping board. The AD8220 requires a ±5 V DC power supply.
The ±5V DC source in the BC circuit comes from a ±5V power supply circuit. It consists of two 9V batteries and two voltage regulators. The NTE977 regulates the +9V power supply to +5V, while the NTE1917 regulates the -9V supply to -5V. The overall ECG signal conditioning circuit is shown in Figure 7. The high-pass, the low-pass, and the notch filters were connected in cascading series. The ±5V power supply circuit is located between the right leg drive board and the USB6009. All circuits and evaluation boards, along with the USB 6009 data acquisition device, were mounted on a 40.64 cm x 20.32 cm base board made of static-dissipative clear cast acrylic sheet. Each circuit or module, as well as their associated components can be easily changed or modified to adapt to different needs for different biosignals.
ECG Signal testing using the designed BC circuit
The designed BC circuitry was tested on a real ECG signal from a human subject. The ECG was recorded by the three Ag-AgCl electrodes attached to the body and sent to the right leg drive circuit. It then passed through the IA, the HPF, the LPF, and finally the notch filter for a series of conditioning. The ECG output at each stage was recorded, displayed, and examined. In order to examine and display the filtering result at each stage, a USB6009 unit and LabView program20 were used for data acquisition, analog to digital conversion, and display. The output of each filter was captured at the VOUT pin located at the top edge of each filter board and was connected to one of the terminals on the USB6009 unit. Figure 8 shows the ECG outputs at each filtering stage. The instrumentation amplifier first amplified the original ECG signal to raise its voltage level (with a gain of 19). At this stage, no visible ECG peaks were observed. After the high-pass filter and further amplification, the “bump” features of the ECG signal started to appear, although there was high frequency noise riding on the ECG output. The subsequent low-pass filter attenuated the high frequency component. However, 60 Hz power line interference was still visible. At this stage, the ECG peaks – P, R, S, T – became more distinct. The notch filter was able to remove the power line noise, resulting in a very clear and smooth ECG signal with P, Q, R, S, T peaks even more distinguishable. This final output of the ECG waveform suggested that the designed ECG conditioning circuit functioned properly and the results are comparable with commercial products.
The sequential usage of the IA, the HPF, the LPF, and the notch filters have resulted a successfully conditioning a raw ECG wave from a human subject. The merits of this circuity not only lie its excellent functionality, but also its flexible modularity. Each filter unit, as well as their associated components, can be easily changed or modified to adapt to other biosignals with different amplitudes and frequencies. The system could also be used to condition other non-biosignals. The device provides a useful educational platform for students to learn, step-by-step, signal conditioning principles, function and construction of active and passive filters, electronic components, interface, and LabVIEW programming tools.
None.
The author declares no conflict of interest.

©2017 Du, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.