International Journal of
eISSN: 2573-2838


Short Communication Volume 5 Issue 2
Department of Chemical Engineering, National Cheng Kung University, Taiwan
Correspondence: Yi-Je Juang, Department of Chemical Engineering, National Cheng Kung University, No. 1 University Road, Tainan, Taiwan, Tel +886-6-2757575 ext 62653
Received: February 24, 2019 | Published: March 8, 2019
Citation: Lin L, Juang YJ. Copolypeptide-assisted fabrication of centimeter long gold nanowire array. Int J Biosen Bioelectron. 2019;5(2):33-36. DOI: 10.15406/ijbsbe.2019.05.00149
In this study, a relatively simple and low-cost method based on micro molding in capillaries was demonstrated to fabricate an array of long, gold nanowires. Owing to its reducing capability, the copolypeptide solution was used to generate gold nanoparticles (Au NPs). The Au NPs/copolypeptide solution was then filled in the microchannels, followed by evaporation and sintering process. It was found that the height of the gold nanowires increased as the solution concentration increased while the width remained similar around 50nm. By using 8:1 (lysine: tyrosine) block copolypeptide, the gold nanowires with 60nm in height, 40nm in width and 1cm in length was obtained.
Keywords: microfluidics, nanowire, nanoparticle, polypeptide, evaporation
Au NPs, gold nanoparticles; Lys, lysine; Tyr, tyrosine; PDMS, polydimethylsiloxane; BSA, bovine serum albumin; MIMIC, micro molding in capillaries; SEM, scanning electron microscope; AFM, atomic force microscope; TEM, transmission electron microscope
One-dimensional (1D) nanostructures, such as nanowires and nanotubes are of tremendous interest for scientific and engineering communities for their unique optical, electrical, mechanical and chemical properties, as well as their potential implementation as devices. Their applications range from semiconductor electronics, biological and gas sensors, environmental science to medical diagnostics.1‒3 A myriad of methods have been proposed and demonstrated to fabricate 1D nanostructures such as electron beam,4 focus ion beam,5 chemical synthesis,6 template-directed synthesis,6 electrodeposition approach.7 nanotransfer edge printing,8 solution evaporation or flow inside microchannels,9,10 adhesion lithography,11 edge lithography or patterning12,13 and so on. Recently, we proposed a relatively simple and low-cost method based on MIMIC to construct an array of a long BSA line structure14 This was achieved by evaporating BSA solution inside microchannels where nearly all the BSA was deposited at the bottom corners of the microchannels, resulting in a long BSA line structure. From the literature, it was found that peptides like tyrosine or poly-L-tyrosine can serve as a reducing agent for formation of Au NPs under alkaline conditions.15‒18 Therefore, in this study, we proposed a method to construct long, gold nanowires by performing MIMIC with usage of polypeptide (or copolypeptide) solution, followed by solution evaporation and sintering process. Effect of polypeptide properties and concentrations, reducing time, micro channel dimension, etc. on nanowire formation were discussed.
The amino acids used in this study, Ne-Z-L-lysine (~99%, Aldrich, Z: carboxybenzyl) and O-benzyl-L-tyrosine (>99%, Bachem), were used as received from Aldrich and Bachem, respectively. THF (ACS Reagent, Merck) and Diethyl ether (Anhydrous, ACS Reagent, J.T. Backer) were dried using Na metal. Hexane (ACS Reagent, EM Science) was dried using calcium hydride. Triphosgene (98%, Merck), 2,2’-bipyridyl (99+%, Aldrich) and bis(1,5-cyclooctadiene) nickel (0) (Aldrich) were used as received. 1.0M HCl in diethyl ether and 33 wt% hydrogen bromide (HBr) in acetic acid were used as received from Aldrich. Iodotrimethylsilane (Me3SiI, 97%, saturated with Copper) was used as received from Alfa Aesar. Polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning) and HAuCl4 were purchased from Aldrich.
Peptide synthesis
The polypeptide synthesis was carried out through polymerization of Ne-Z-L-lysine (Z-Lys) and O-benzyl-L-Tyrosine (Bn-Tyr) NCAs using the zero valent nickel initiator, 2,2’-bipyridyl-Ni(1,5-cyclooctadiene) (BpyNi-COD). The detailed procedures can be found in the literature.19‒21 Poly-L-lysine-b-poly-L-tyrosine was de protected by using HBr and Me3SiI, followed by hydrogenation in methanol using Pd/C to ensure the complete de protection. The notation used for the synthesized polypeptide throughout is Lysm-b-Tyrn, where m and n are the number of amino acids in one chain. In this study, the Lys-Tyr block copolypeptides with m equal to 4 and 8, and n=1 have been synthesized for usage.
Fabrication of metallic nanowires
A Lys-Tyr block copolypeptide aqueous solution (>1 wt %, pH=7) mixed with an Au (III) precursor solution was aged for 2 hrs to ensure the complete of reaction. A droplet of the resultant solution, i.e. Au NPs/polypeptide solution, was dispensed at one end of the microchannels which were formed by conformal contact between a PDMS micro mold and a cleaned glass slide. The width, height and length of the microchannels were 100mm, 4mm, and 1cm, respectively. The channel wall to wall distance was 100mm. The microchanels were filled within minutes due to capillarity. The solution-loaded micro channel array was allowed to evaporate at the ambient condition for 2 hrs. Upon solvent evaporation and removal of PDMS micro mold, the polypeptide line structures on the glass substrate was placed in the high temperature furnace (DF-202, Mandarin Scientific Co., Ltd, Taiwan) with temperature set at 500oC. The Au NPs were analyzed by UV-Vis and TEM (Hitachi H7500). The line structures were observed and characterized by optical microscope (TE-2000-S, Nikon) and SEM (JEOL JSM-6700). Characterization of the gold nanowires was conducted by SEM and AFM (PicoPlus 5500, Agilent Technologies, Inc.).
As mentioned previously, both tyrosine and poly-L-tyrosine can reduce gold ions to form Au NPs. Hence, the solutions of both peptides were used for initial feasibility study. Although the solutions filled the microchannels completely within seconds, deposition of tyrosine (or poly-L-tyrosine) could not form a continuous line structure. However, it was found that poly-L-Lysine solution filled the microchannels easily and a continuous line structure was formed. Since poly-L-Lysine does not possess the reducing capability, therefore, both 4:1 and 8:1 (Lys:Tyr) block copolypeptides were synthesized in this study. When dispensing the copolypeptide solution at one end of the microchannels, it filled the microchannels and formed a continuous line structure after evaporation as shown in Figure 1. The line structures were spaced by 100mm, corresponding to the spacing between channels and the width of channels. Since 8:1 has better solubility in DI water and is less viscous than 4:1, it was used for subsequent experiments. For solution to fill the microchannels spontaneously by capillarity, the dimension of microchannels is crucial. That is, the aspect ratio of the microchannels needs to be properly designed, which can be determined by the following equation22
where Pc is the capillary pressure, g is the surface tension, a is the channel height, b is the channel width, and q is the contact angle. It was calculated that the aspect ratio needs to be less than 0.36 in order for the solution to fill the microchannels spontaneously (the aspect ratio is 0.04 in this study). The solution indeed could not fill the microchannels when their aspect ratio was 1 as we tested. The plasma treatment was applied to the surface of PDMS microchannels and the solution filled the microchannels readily, however, the deposited line structure was detached from the substrate as the PDMS mold was removed.
The advantageous benefit of using the block copolypeptide is that solution with higher concentration (>1 wt %) can reduce gold ions in several minutes at neutral condition, which is different from using tyrosine or polytyrosine at the alkaline condition and lower concentration. Formation of Au NPs was evidenced by a distinctive UV absorption with a maximum centered at around 520nm as shown in Figure 2A. The time-resolved UV-vis measurements were performed and the corresponding absorption at 600nm as a function of time is shown in Figure 2B. The time course showed a rapid growth of gold nanoparticles between 0 and 20mins, followed by a plateau signaling the end of reaction. From the TEM results, it was found that size distribution of the gold nanoparticles became broader as the reaction time increased. For 120-min reaction time, the size distribution was from 10 to 150nm.
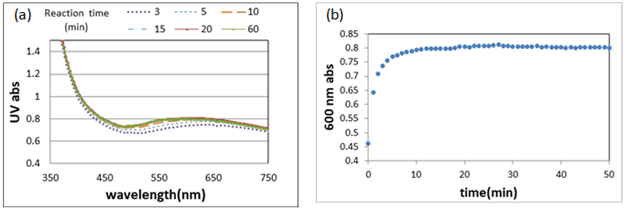
Figure 2 (A) The UV-vis spectra for different reaction times (B) The time-resolved UV-vis measurements. 3wt% solution was used.
Figure 3A shows the cross-sectional view of the line structure. Similar to our previous study, a triangular-like shape was observed. The height of these line structure (measured from the bottom to the tip) increased as the concentration of the copolypeptide solution increased, which is shown in Figure 3B. It is worthy of mentioning that when the concentration was less than 1wt%, the continuous line structure could not be formed. On the other hand, as the concentration exceeded 6wt%, the solution became much more viscous that the solution could not fill the microchannels. After the sintering process, the copolypeptide was removed and the nanowire array was formed due to the fusion of Au NPs. Figure 4 shows the SEM images of the gold nanowires on a glass substrate obtained from using 1, and 3, and 6 wt% of copolypeptide solutions. For 1wt%, part of the line structure did not seem to be clearly formed. Despite different solution concentrations, the width of the nanowires was approximately 50nm. However, the height of the nanowires increased when the concentration increased as shown in Figure 5. As to the reaction time, there seems to be no influence on the height of the copolypeptide line structure and the width of the nanowires as shown in Figure 6A & Figure 6B, respectively when 3wt%

Figure 3 (A) The cross-sectional view of the line structure. (B) The height of copolypeptide line structure. The reaction time is 120min.

Figure 4 The nanowires obtained by using (A) 1, (B) 3, and (C) 6 wt% solution concentration. The reaction time is 120 min.
In this study, we have demonstrated a relatively simple and low-cost method to fabricate an array of long, gold nanowires. It is necessary to use copolypeptide solution (i.e. lysine-b-tyrosine in this study) instead of tyrosine or polytyrosine because it not only retains the reducing capability but also form a continuous line structure after evaporation. For spontaneous filling of the microchannels, its aspect ratio needs to be less than 0.36. The size distribution of the nanoparticles ranges from 10 to 150nm. The height of the gold nanowires increased as the solution concentration increased while the width remained similar around 50nm. By using 8:1 (lysine: tyrosine) block copolypeptide, the gold nanowires with 60nm in height, 40nm in width and 1cm in length were obtained.
Ministry of Science and Technology in Taiwan (MOST 107-2221-E-006-103-MY3).
The authors would like to thank Prof. Jeng-Shiung Jan from Department of Chemical Engineering at National Cheng Kung University for providing polypeptides.
Authors declare that there is no conflict of interest.

©2019 Lin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.