International Journal of
eISSN: 2573-2838


Mini Review Volume 7 Issue 3
1Department of Health and Safety, Moscow State Pedagogical University, Russia
2Department of Water Resources and Aquaculture, Kazan State Energy University, Russia
3Department of Natural Sciences, Reawis Medical University, Russia
Correspondence: Petrov Sergey, Department of health and safety, Moscow State Pedagogical University, Russia
Received: April 29, 2021 | Published: May 31, 2021
Citation: Sergey P, Anatoli L, Sergey L. Antioxidant activity of the «litar» material. Int J Biosen Bioelectron. 2021;7(3):91-94. DOI: 10.15406/ijbsbe.2021.07.00219
The antioxidant status of the body is a very important indicator of health. It depends on the ability of body fluids to suppress free radicals. Therefore, the study of methods for analyzing body fluids to determine the total antioxidant capacity (TAC) and total antioxidant activity (CAOA) of the body are very important for medical practice. The composition of urine reflects changes in the body, diet and the content of polyphenol metabolites. At the same time, urine collection is a non-invasive method of analysis, which simplifies the determination of the antioxidant status of the body. The purpose of this work is to describe a non-invasive method for determining the antioxidant status of the body by the total antioxidant activity of urine using potentiometric coulometry on the example of “LitAR,” a nanosized composite implantation material, approved for use in medical practice.
Keywords: the total antioxidant activity, antioxidant status of the body, total antioxidant activity of blood, saliva and urine, human oxidativestress biomarkers, nanoscale composite «LitAr», urine, coulonometric analysis metod
To characterize antioxidant substances, the concept of “total antioxidant capacity” (TAC) was introduced.1–3 Oxidative stress is an imbalance between Reactive Oxygen Species (ROS) and human antioxidant defense. This risk factor arises under the influence of xenobiotics and endogenous processes. To assess the antioxidant status in vivo, it is necessary to take into account the synergistic interactions between antioxidants. Saliva analysis depends on oral hygiene and dental health. Urine samples are obtained non-invasively and this simplifies the body antioxidant status study. The composition of urine reflects changes in the body, diet and the content of polyphenol metabolites.4 To assess the ability of liquids to inhibit free radical reactions, we will use the term CAOA - “cumulative antioxidant activity”2 CAOA can be one of the criteria for monitoring of individualized therapy and evaluating the treatment of a pathological process, in the development of which processes of peroxidation can be noticed.5 When testing the effect of medicinal substances on urine CAOA, numerous data on the effect of food and beverages on its level have been accumulated. Walnuts and cocoa increased urine TAC 6 hours after taking.6–8 A decrease in urine TAC level was found after drinking of blackberry juice.9 But it was increased after eating spinach, strawberries10 cherries11 and drinking drinks based on a mixture of apple, grape, blueberry and pomegranate juices and grape skins, grape seeds and green tea extracts as well as while eating a fatty meal.12 A drink based on a mixture of pineapple, blackcurrant and plum juice did not affect both urinary TAC and plasma TAC.12 Drinking of grape juice during 5 days increased urinary TAC.13 An increase in TAC was found when vitamin C (870mg/ l) was added to tomato juice.14 After a single or 2-week drinking of green or black coffee, the urine TAC level did not change.16,17 The purpose of this work is to describe a non-invasive method of determining the antioxidant status of the body by the total antioxidant activity of urine using potentiometric coulometry on the example of "Litar," an implantation material - a nanosized composite which is approved for use in medical practice.
“LitАr” is a material (Figure 1) and is a mixture of biopolymer (collagen or calcium-sodium alginate) and calcium hydroxophosphate, also called hydroxylapatite, hydroxyapatite or simply apatite. Figure 2 shows:
This is what provides the clinical effect: while the regeneration of conventional resorbable materials lasts months and years, it takes weeks for “LitAr.” During the first 10-15 days, the implanted material is bi-transformed and gradually turned into the type of tissue (bone, cartilaginous or parenchymal) that was before the disease in the defective area of human tissue.15,18 The material can be administered either orally or by means of injection. When injected, the expected result is reliably predicted. Oral administration outcome is more difficult to predict if there are gastrointestinal, diabetic and vascular problems.
The study involved 3 participants: 2men aged 67 and 75 years old and a woman 25 years old. They were practically healthy people, mainly of mental labor, who have not been sick for 3 months. The study was carried out under the ethical principles of the Helsinki Declaration of the World Medical Association. Control samples of morning urine were collected on an empty stomach at 7o'clock according to the Nechiporenko’s method, after which “LitAr” (0.5g) was taken. Urine samples were also collected on an empty stomach at 8 o'clock. Samples were analyzed without processing within an hour after collection. Evening urine samples were collected at 8p.m. or 9p.m. after taking “LitAr” (0.5g) at 7p.m., at that they were stored in a refrigerator at + 6°C before analysis. A total of 30 urine samples with CAOA ranging from 285 to 1647 mg rutin (Ru) per 1 dm3 (liter) were examined. The study results were determined by the effects of synergism (increase in CAOA) and antagonism (decrease in CAOA). CAOA indicators of evening urine (CAOA evening) taken at 8 p.m. relative to the control levels (CAOAcontr.) of morning urine samples taken at 7a.m. on an empty stomach according to the Nechiporenko method (Figure 1) were determined in relative percentage (%rel.) according to the following formula: CAOA=100 (CAOAevening. - CAOAcontr.): CAOAontr. The hydrogen ions (pH) and the redox potential (Eh) activity indicators were measured on the I-160MI laboratory ionometer.
The samples CAOA levels were determined according to our certified method of the coulometric analysis by using electro generated bromine radicals done on an automated, certified, serial coulometer “Expert-006-Antioxidants” made by “Econix-Experts Ltd” (Moscow).19 The determination of CAOA levels was carried out in terms of a standard sample Ru level in mg. per 1dm3 of urine samples without conservation. The device was calibrated with an alcohol solution of the Russian standard sample (RSS) of Ru20 prepared according to the current State Pharmacopoeia of the XIth edition. The obtained results statistical processing was carried out through a modal value (mode) of 10 determinations,21 the relative error in determining the CAOA of the whole urine (E rel.) was within 0.8-1.5%. Oxidized samples (CAOoxid.) synengism and antagonism effects (CAOA) indicators in relation to the initial urine samples levels (CAOAorigin.) were determined in % rel. according to the formula: CAOA=100 (CAOAacid. - CAOA origin): CAOA origin. The urine samples were oxidized with 3% medical hydrogen peroxide (HP) produced by Iodine Technologies and Marketing Ltd. (Moscow, Russia) at a ratio of 1: 1 by volume. An increase in CAOA levels is not always a favorable sign. An increase in the CAOA level may indicate of the oxidative stress developing, accompanied with the mobilization of the antioxidant system. Uric acid (the main antioxidant of blood plasma which contribution is about 50% of CAOA)22 level increasing causes CAOA level increasing, and as in the case of renal failure with uremia, for example, causes oxidative damage to proteins and lipids and decreases the ascorbic acid concentration.23,24 Both the release of molecules of average weight25 or cell contents during necrosis and increase in the blood bilirubin level are also accompanied with CAOA level increase.
The study of HIV-infected patients CAOA levels done by us showed that the CAOA index of whole blood, which is significantly lower than in healthy people with minimal levels in the early stages of the disease is a more informative indicator of HIV-infected patients CAOA level, at that sick women CAOA blood level is reliably lower than that of men.26 Taking of 0.5gr. of composite "LitAr" twice a day by a patient (67 years old) at 7a.m. and 7p.m. caused the effects of synergism (increase in CAOA) and antagonism (decrease in CAOA). Evening urine CAOA indicators (CAOAevening) taken at 8 p.m. in relation to the control levels (CAOAcontr.) of morning urine samples taken at 7 a.m. on an empty stomach according to the Nechiporenko method (Figure 3) in% rel. are shown in Figure 3. The effects of synergism (increase in CAOA) and antagonism (decrease in CAOA) were also revealed when a patient (a 25-year-old woman) took the composite "LitAr" (0.5g) twice a day at 7a.m. and 7p.m. CAOA of evening urine indicators (CAOAevening) taken at 8 p.m. in relation to the control values (CAOAcontr.) of morning urine samples taken at 7 a.m. on an empty stomach according to the Nechiporenko method in% rel. is shown in Figure 4.
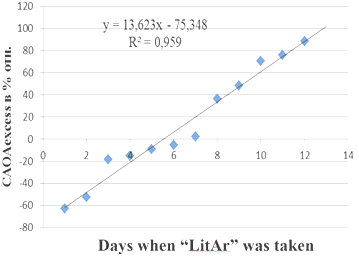
Figure 3 Increase and decrease in the cumulative antioxidant activity (CAOA) of evening urine indicators (CAOAevening) in relation to the control values (CAOAcontr.) of morning urine samples taken according to the Nechiporenko method in relative percentages during the period of taking the “litar” composite.
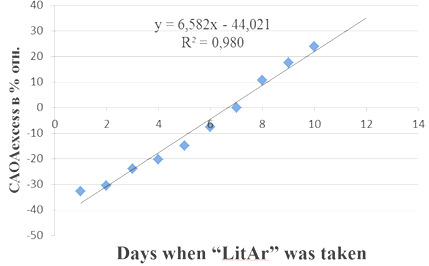
Figure 4 Increase and decrease in the cumulative antioxidant activity (CAOA) of evening urine indicators (CAOAevening) in relation to the control values (CAOAcontr.) of the woman’s morning urine samples taken by the Nechiporenko method in relative percentages during the period of taking the “litar” composite.
Human biofluids CAOA studies have both diagnostic and prognostic significance. They are becoming popular and may become standard laboratory tests. Human urine (TAC) studies have shown that these markers can be useful in assessing the antioxidant status of the body under the influence of diet, diseases and environmental conditions of human life. The effects of synergism (increase in total antioxidant activity) and antagonism (decrease in total antioxidant activity) of the relative levels of evening urine in the relation to the control values of morning urine taken on an empty stomach were revealed after patients’ taking of 0.5g. of the "LitAr" composite in the morning and in the evening. For the first time, the effects of synergism and antagonism of the relative indicators of morning and evening urine samples that were oxidized with 3% medical hydrogen peroxide were revealed.
None.
The authors declare that there is no conflict of interest.

©2021 Sergey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.