International Journal of
eISSN: 2573-2838


Review Article Volume 5 Issue 4
Department of Biomedical Engineering, Sheikhbahaee University, Iran
Correspondence: Hamidreza Shirzadfar, Department of Biomedical Engineering, Sheikhbahaee University, Isfahan, Iran
Received: July 23, 2019 | Published: August 7, 2019
Citation: Shirzadfar H, Amirzadeh P, Hajinoroozi MH. A comprehensive study over the jaundice causes and effects on newborns and reviewing the treatment effects. Int J Biosen Bioelectron. 2019;5(4):107-112. DOI: 10.15406/ijbsbe.2019.05.00162
60% of normal newborns become clinically jaundiced during the first week of life. Unconjugated (indirect) hyperbilirubinemia occurs as a result of excessive bilirubin formation and because the neonatal liver cannot clear bilirubin from the blood rapidly enough. Although most newborns with jaundice are otherwise healthy, they need to be monitored because bilirubin is potentially toxic to the central nervous system. Sufficiently elevated levels of bilirubin can lead to bilirubin encephalopathy and subsequently kernicterus, with devastating, permanent neurodevelopment handicaps. Fortunately, current interventions make such severe sequelae rare. But because neonatal jaundice is so common, many infants—even those who are unaffected—are monitored and treated to prevent substantial damage that would otherwise occur in a few cases.
Keywords: jaundice, unconjugated hyperbilirubinemia, toxic; bilirubin, bilirubin encephalopathy, kernicterus, neonatal jaundice
Bilirubin is a yellow colored substance produced in hemoglobin metabolism. Initially, hemoglobin is broken to heme and globin. Heme is a composite of blood that causes the exchange of oxygen. Hem oxidizes to Billy Virden and eventually becomes Billy Rubin.1 On the other hand, the globin converts to amino acids. Bilirubin is a toxic substance that should be excreted from the body and is usually excreted via the liver and kidneys. Bilirubin - Albumin complex is called the indirect or non-binding bilirubin that is fat-soluble and can pass through the layers of fat that is present in the baby's brain and damage the baby's brain.2 Conjugated bilirubin, called direct bilirubin, is water soluble that passes to the liver and is excreted in stages, and much of it is excreted in the feces, and a small portion is excreted through the urine.3 Conjugated bilirubin is also known as direct bilirubin which is water soluble. A portion of conjugated bilirubin is excreted in urine then the remainder is secreted into bile and then into small intestine. In gastrointestinal tract, in the terminal ileum and colon, bilirubin is de-conjugated by bacterial enzymes and metabolized to urobilinogen.4 The 18 percent of urobilinogen is absorbed via entero-hepatic circulation and delivered back to liver. 80% of urobilinogen is converted into stercoblin and excreted in feces. Stercoblin gives characteristic color of feces. 2% of urobilinogen is converted into urobilin and excreted via the urine. Urobilin gives the characteristic color of the urine (Figure 1).5,6
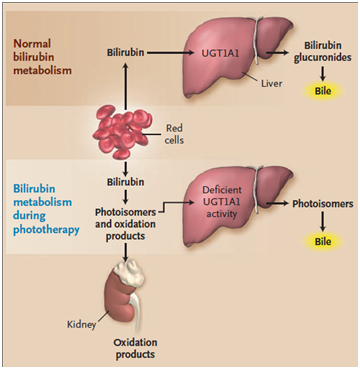
Figure 1 Bilirubin metabolism.6
Diagnosis of bilirubin level
As you can see in Figure 2, the human body can be divided into 5 regions: palms, soles, lower trunk and thighs, upper body and arms, head and neck. The cause of jaundice is the bilirubin. This bilirubin can be diagnosed as an unrelated bilirubin to albumin that circulates around the bloodstream.7 This rule represents the predictive value of the concentrating of the total serum bilirubin density (TSB) for any area that is yellow and contains jaundice. When the first yellow fever starts to reach the rest of the head, it indicates an increase in TSB.8 Each region shows the level of jaundice in three modes: normal, moderate and critical cases. Kramer’s rule is generally used by the Pediatrician to predict the value of TSB concentration based on the physical color of the skin. For example, if the jaundice of the skin reaches the upper body, TSB>15mg/dl, under such conditions the baby should be treated by the phototherapy light. Although this method is non-invasive, in some cases, when the bilirubin level exceeds more than 15mg/dl, other devices and methods for diagnosis are required.9 The reasons of bilirubin increment are very different, but one of these reasons is the destruction of red blood cells. Normal life of the red blood cells is 120days, but if it is destroyed at once, the bilirubin will increase. The reason of the destruction of the white blood cells is the discrepancy between the baby and mother's blood RH. On the other hand, the blood zones in the baby's brain are not completely formed, and the indirect bilirubin, lipid-soluble, passing through these areas can cause paralysis or other brain problems.10
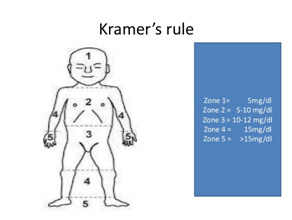
Figure 2 Kramer’s rule in jaundice spread in an infant’s body.8
Materials used
The purpose of the bilirubin test
The goal of the bilirubin test is to investigate the liver function and the diagnosis of the liver diseases, such as hepatitis or cirrhosis, and also to diagnose and evaluate the effects of a medicine that damages the liver. It is Also used to find things that block the bile ducts, such as rocks, pancreatic tumors, or other conditions.11 This test can provide conditions for detecting any increase in the destruction rate of the red blood cells, such as hemolytic anemia or hemolytic disease in a newborn. It also helps us to determine whether the baby needs treatment via the phototherapy, or the baby's jaundice is too critical and requires blood replacement or transfusion, which is a rare case.12,13 There are some issues that should be noted for adults before this experiment, but for children, no special preparation and special care is necessary. Adults should avoid eating and drinking 4 hours before the test. If you are using drugs or you are sensitive to drugs or even if you are pregnant, you should be closely supervised by the doctor and you should carefully read and fill out the test information forms to understand the significance and benefits of this test.14 This test is done in two ways: heel and tip vein sampling.15
Sampling from the heel
In this method, a few drops of blood are taken from the heel of the baby. It should be noted that the heel skin should be cleaned with alcohol before the sampling and then a small hole should be made with the help of a sterile lancet, and after the sample is collected, the place must be cleaned with a gauze or cotton.15 Some hospitals and healthcare centers use a device called the Bilirubin meter, which is a small handheld device.16 If this device is used, no blood sampling is needed, as it is a screening test. When the baby's jaundice is high, Blood sample is required.
Sampling of veins
This sampling is done by a professional health specialist. After wrapping an elastic band around the arm and cleaning the site, a blood sample is collected by a needle and the necessary examinations are done in the following steps.16 While sampling the heel, the baby may feel a little pain, but during the vein sampling since an elastic band is wrapped around the arm, the baby may not feel any pain caused by the needle. Table 1 shows the normal level of bilirubin in adults that has been studied in three modes: total, direct and indirect:
Bilirubin levels in adults |
|
Bilirubin type |
Bilirubin level |
Total bilirubin |
0.0-1.4mg/dL or 1.7-20.5 mcmol/L |
Direct bilirubin |
0.0-0.3mg/dL or 1.7-5.1 mcmol/L |
Indirect bilirubin |
0.2-1.2mg/dL or 3.4-20.5 mcmol/L |
Table 1 Bilirubin levels in adults.17
Commonly used Methods to measure direct-reacting bilirubin (DBIL) are based on either the diazo reaction or direct spectrophotometry. DBIL values obtained by diazo procedures vary with the concentration and type of the diazo reagent, the pH of the reaction mixture and the duration of the reaction. These factors are mainly responsible for the large variability in DBIL values obtained for specimens analyzed by different methods. The non quantitative reaction of bilirubin diglucuronide (dB), bbilirubin (Ba), and pure ditaurobilirubin (D’FB) in the direct diazo assay has been amply documented. Direct spectrophotometry is not affected by the variables associated with diazo assays, but it is available only to those who possess a certain type of instrumentation.17,18
For phototherapy the Green-blue light is effective in the range of 460-490nm. The absorption of light by natural bilirubin causes the formation of isomers of configuration, structural isomers and oxidation products.19 The configurational isomers are faster and more reversible than the structural isomers. Structural isomers are slow and irreversible. Photo oxidation slowly occurs by any of the two structural and configuration isomers, and photo oxidation products are mainly excreted in the urine (Figure 3).20 The absorption of light by the normal form of bilirubin (4Z, 15Z-bilirubin) generates transient excited-state bilirubin molecules. These fleeting intermediates can react with oxygen to produce colorless products of lower molecular weight, or they can undergo rearrangement to become structural isomers (lumirubins) or isomers in which the configuration of at least one of the two Z-configuration double bonds has changed to an E configuration. (Z and E, from the German zusammen (together) and entgegen (opposite), respectively, are prefixes used for designating the stereochemistry around a double bond. The prefixes 4 and 15designate double-bond positions). Only the two principal photo isomers formed in humans are shown. Configurational isomerization is reversible and much faster than structural isomerization, which is irreversible. Both occur much more quickly than photo oxidation. The photo isomers are less lipophilic than the 4Z, 15Z form of bilirubin and can be excreted unchanged in bile without undergoing glucuronidation.19,21 Lumirubin isomers can also be excreted in urine. Photo oxidation products are excreted mainly in urine. Once in bile, configurational isomers revert spontaneously to the natural 4Z, 15Z form of bilirubin. The graph, a high-performance liquid chromatogram of serum from an infant undergoing phototherapy, shows the presence of several photo isomers in addition to the 4Z, 15Z isomer. Photo isomers are also detectable in the blood of healthy adults after sunbathing.19,22

Figure 3 Mechanism of Phototherapy.19
Function of non-invasive optical devices
Absorption is carried out in the range of 300 to 900 nm, and the results of this study give us information about the skin metabolites.23 Melanin, which is in the epidermis layer and it is the main pigment of the skin and hair, is the main cause of light obstruction.24 Hemoglobin also absorbs light. As you can see in Figure 4, the bilirubin has a range of 400 to 520nm, while hemoglobin has a different spectrum. In the area of 400 to 800nm, the main factor is the light absorption of melanin, and hemoglobin and bilirubin are not on the melanin pathway.25 Due to the bilirubin level in the blood, light is absorbed or reflected. The greater the concentration of bilirubin in the blood is, the greater the absorption occurs.26 The reflected light from the solution is then converted into a voltage by the photodiode. In Figure 5, a set of values is obtained that correlates between the concentrations of the bilirubin in the solution and the output voltage of the diode.27 Measuring the amount of jaundice in a tissue underneath the baby's skin is determined by measuring the difference in optical density for light in a blue (450nm) and green (550nm) wave.28 Measuring probes have 2 optical pathways, and this method is able to accurately measure the jaundice in the infant's substrate tissue, as a result the effects of melanin pigmentation and skin maturity are minimum.29 When the probe is placed on the spine or the baby's forehead, the xenon lamp flashes and the light illuminates the skin. Dispersed light is absorbed repeatedly in the skin and the subcutaneous tissue, and then reaches the glass fiber sensor. The light is scattered in regions that have less depth, but in areas with higher depth, it passes through the tissue and reaches the photodiode.30 Absorbed light intensity formula, based on the evaluation of the CB bilirubin density, is as follows:31
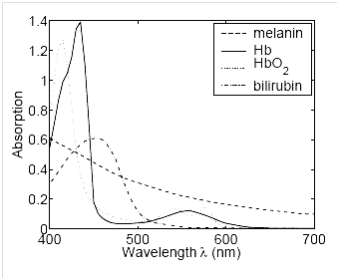
Figure 4 Absorption vs. wavelength graph of hemoglobin, melanin and bilirubin.25
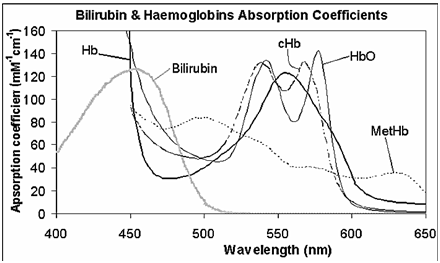
Figure 5 Absorption spectra for Hemoglobin, & Bilirubin in the 400-650nm range.29
(1)
Where is the intensity of the light, x is the optical path, k is the coefficient which depends on the material concentration and the wavelength, and L(x) is the light intensity at x.32 At a similar wavelength to the hemoglobin, the light is absorbed by melanin and the hemoglobin. Spectral Absorption Characteristics of Bilirubin and Hemoglobin are measured with different wavelengths Land L. Two intensity of L () and L () give us the possibility to calculate the bilirubin concentration, which is as follows:33
(2)
Where is the tuning factor and and are the coefficients of weight that should be experimentally evaluated. Wavelengths are the compromises between the necessity of measuring the maximum hemoglobin and the bilirubin and the necessity of emitting the similar beams in the tissues:33
Billy check
Billy Check is a simple method that can help to measure the skin’s total serum bilirubin level in a non-invasive and fast way.34 In this section, we want to compare the measured serum bilirubin by Billy Check and the laboratory method. Jaundice occurs in approximately 60% to 80% of premature or mature infants, and between 6% and 7% of mature infants have higher than 12.9 mg/dl of jaundice and less than 3% of them have above 15mg/dl of jaundice.35 In order to take appropriate treatment for newborns, it is necessary to evaluate bilirubin in three ways: ocular, skin and the head. The method for measuring serum bilirubin (TSB) is a method that has problems such as infection and anemia, because many samples should be taken from the newborn baby, as well as being a costly method. One of these methods is the eye evaluation, which has two basic problems.36 First, this is an experimental practice, and the more the physician's experience is, the more appropriate the diagnosis will be and there is no reliable measure for this method, secondly, the estimates are based on the jaundice in the cephalocumulus, in this method the jaundice in the head and the face is equivalent to 5, in the abdomen is 15 and in the legs it is more than 20. This method is not suitable for infants who are treated with phototherapy.37 It should be noted that factors such as the color of clothing and the light of the examination environment can also affect this diagnosis. In order to increase the accuracy of diagnosis, non-invasive methods of blood bilirubin assessment were presented, one of which is TCB.38 According to the studies, there is a solid and coherent relationship between the skin measuring method of bilirubin and its laboratory method, and the use of bilirubinometery can replace the laboratory method, but in special cases and in infants with high jaundice, it is necessary to check the serum level.39 The result is a defined relationship between the serum bilirubin value and the yellow light wavelength obtained from the infant's skin, which is expressed by the following equation:40
(3)
Where M is the average wavelength of the infant’s yellow color skin and TSB is the total serum bilirubin. This relationship indicates that, for the light spectra above 140nm, the serum bilirubin level is directly related to the wavelength of the yellow light.40
Evaluation of newborn jaundice diagnostic modeling
In general, jaundice is of two types: physiological and pathological. Physiological jaundice occurs for various reasons such as stroke during the labor, blood clotting and slipping of the blood cells, premature birth and breastfeeding issues, usually seen 2-3days after birth and disappearing until the seventh day.41 In preterm infants, the intensity of jaundice is higher, so it appears on day 3 and 4 and disappears after the fourth day. This type of jaundice is treated on its own and does not require any treatment.42 The second type is pathological jaundice which occurs for some reasons including enzymatic defects such as G6PD enzyme deficiency and maternal and neonatal blood malformation and infection.43 The main cause of pathological jaundice is maternal and fetal blood incompatibility, which can be due to the difference in RH, so that the mother has negative RH and the infant has positive RH or even the blood types, especially when the maternal blood type is O and the neonate’s blood type is A and B, All of which causes the red blood cells to collapse and the baby is not able to remove this large amount of bilirubin out of his or her body.44 Factors like having jaundice in the first 24 hours of birth, bilirubin levels greater than 12mg/dl in term neonates, increasing bilirubin levels at a rate greater than 5 mg /dl, an increase of more than 10-14mg/dl in premature infants, all Indicate the pathological jaundice.45 For the treatment of this type of jaundice, phototherapy is used and in cases of severe jaundice, blood exchange is required. In the presentation of the diagnostic scheme, which is described in the following, we use important factors that separate these two types of jaundice.46 The method for detecting databases is that laboratory samples taken from the infants were collected and 145 newborns were considered for this study, in which the cases of 25 newborns were defective and eliminated, and eventually the characteristics of 120 newborns was applied in the model.47 Sufficient information from the infant, such as the amount of bilirubin in the blood serum, the day of affecting to jaundice, etc., and information about their family, such as having jaundice in their infant's siblings was also collected.
Comparison of the effect of normal blue and white light on the reduction of serum bilirubin
For this study, 50 neonates with jaundice were studied and none of them had a history of certain diseases. When all babies passed 2 to 7days of their birth, they were treated with normal blue and white light, and those with high bilirubin levels were treated with the blue light.48 Both groups were equal in conditions such as the distance of the light source from the body, body surface, weight, gender and age. Before the phototherapy, 12hours and 24 hours after phototherapy, 2cc of blood samples was taken and then tested. Direct bilirubin levels and total bilirubin levels are measured using standard methods.49 Studies were conducted on comparison of the blue light and the normal white light in neonates with jaundice. Above cases indicate that the obtained amounts are significantly lower than those obtained from phototherapy.50
Bilirubin is normally cleared from the body by hepatic conjugation with glucuronic acid and elimination in bile in the form of bilirubin glucuronides. Neonatal jaundice stems from a transient deficiency of conjugation (exacerbated in preterm infants) combined with increased turnover of red cells. Pathologic conditions that can increase bilirubin production include iso-immunization, heritable hemolytic disorders, and extra-vested blood (e.g., from bruises and cephalhematomas).It is well known that phototherapy is able to reduce serum bilirubin and that the reaction responsible of its effect occurs in the skin. The use of transcutaneous bilirubin measurement during phototherapy has been questioned because shaded skin areas remain icteric while exposed areas are blanched. In this study we discussed the jaundice occurring in newborn infants. Also we explained the procedure of jaundice and the routine of spreading jaundice in the whole body. In addition we studied the methods for testing the serum bilirubin and the process of diagnosis and curing.
None.
None.
Author declares that there is no conflict of interest.

©2019 Shirzadfar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.