eISSN: 2471-0016


Mini Review Volume 4 Issue 4
1Department of Hemodynamics, Americas Medical City, Brazil
1Department of Hemodynamics, Americas Medical City, Brazil
2Department of Hemodynamics, Hospital Samaritano, Brazil
2Department of Hemodynamics, Hospital Samaritano, Brazil
Correspondence: Felippe Dantas Vilela, Member of SBHCI (Brazilian Society of Hemodynamics and Interventional Cardiology) Hospital Samaritano-Setor de Hemodinamica, R. Bambina, 98-Botafogo, Rio de Janeir-RJ, Brasil, Tel +55(21) 98803-5888, Fax +55 (21) 3444-1000 (403)
Received: January 01, 1971 | Published: April 28, 2017
Citation: Vilela FD, Cortes LA, Costa GBF, et al. Transcatheter aortic valve replacement. Int Clin Pathol J. 2017;4(4):98–102. DOI: 10.15406/icpjl.2017.04.00104
About 7% of the population over age 65years suffers from degenerative aortic stenosis (AS). The prognosis of patients with symptomatic severe aortic stenosis is poor without valve replacement, and approximately one third of these patients over the age of 75years are not referred for surgery. Over the past decade, an extremely promising interventional technique entered the scientific community of cardiologists and cardiac surgeons. This technique consists on transcatheter implantation of a bioprosthetic aortic valve in patients with severe aortic stenosis. Today, almost 15years from that first-in-human implantation, the transcatheter aortic valve replacement (TAVR) plays a key role in the treatment of severe aortic stenosis; now considered as being the most effective treatment in inoperable patients and a reliable alternative to conventional surgical aortic valve replacement in high- and intermediate-risk patients. But the success of TAVR in less fragile and less morbid patients still need more studies to be confirmed the long-term durability, and the hemodynamic and clinical gains from TAVR. A newer-generation valves have shown their potential for further improvement in safety profile and overall outcomes. This manuscript is an attempt to review the current knowledge of TAVR.
Keywords: heart valve diseases, heart valve prosthesis implantation, bioprosthesis aortic valve, aortic valve stenosis, transcatheter aortic valve replacement
AS, aortic stenosis; TAVR, transcatheter aortic valve replacement; AVR, aortic valve replacement; STS, society of thoracic surgeons; EUROSCORE, European system for cardiac operative risk evaluation; ACC/AHA, American college of cardiology/American heart association
Approximately 250,000 procedures of TAVR have been performed worldwide in more than 1,000 centers, and about 15,000 patients were randomized in clinical trials showing that TAVR is a respected treatment option for symptomatic patients with severe AS.1 The prognosis of these patients with symptomatic severe AS is poor without valve replacement, and the mortality rate is 50% at 2years.2,3 The aortic valve replacement (AVR) is the only treatment that has proved helpful in increasing survival rates of population and no clinical treatment has shown any efficacy in improving outcomes. Despite the 2014 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for AVR as a class I indication for severe symptomatic AS,4 nearly one third of patients with severe symptomatic AS are not referred for surgical AVR.3 This is often because multiple comorbidities and frailty all result in poor prognosis and high mortality rates for surgical AVR.5 Several new TAVR devices are now being used with modern features that address the limitations of the first-generation devices including paravalvular leak (i.e., lower profile, easier positioning, repositionability and retrievability). In this mini review, we will focus on the most important characteristics of TAVR procedure. Other purpose is to stimulate the development of new trial in the TARV field.
AS can be caused by rheumatic disease or more commonly by calcification of a congenitally bicuspid or trileaflet valve. The prevalence of aortic stenosis increases with age, averaging 0.2% in the 50–59year cohort and increasing to 9.8% in the 80–89year cohort.6 The pathophysiology of AS is a result of an inflammatory process caused by endothelial damage due to mechanical stress, lipid penetration leading to fibrosis, leaflet thickening, and finally calcification.7 Calcific aortic stenosis causes increased leaflet stiffness and a narrowed aortic valve orifice those results in a pressure gradient across the valve.8 The progressive aortic valve narrowing with concomitant left ventricular hypertrophy lead to the classic triad of aortic stenosis symptoms: heart failure, syncope and angina. The symptomatic AS is rapidly fatal;if not treated the annual mortality is 25%, with an average survival of only 2 to 3years.9 One third of these patients over the age of 75years are not referred for surgery. The severity of AS can be assessed by Doppler echocardiography with findings of a maximum aortic jet velocity>4.0m/s, mean transvalvular pressure gradient>40mmHg, or continuity equation valve area <1.0cm2 or valve area indexed for body surface area <0.6 cm2/m2.9 The surgical risk calculators include the Society of Thoracic Surgeons (STS) or the European System for Cardiac Operative Risk Evaluation (EUROSCORE). Both the EuroSCORE and STS score define operative mortality within 30days from operation or later if the patient remains hospitalized.10 Patients are classified as being at high surgical risk if they have an STS score of >8% or logistic Euro SCORE >20%.9 Most recent analysis of STS score performance demonstrates that it is remarkably good at predicting 30-day mortality on a population basic, whereas the logistic Euro SCORE can overestimate the surgery risk.11 The ACC/AHA guidelines for the indications of aortic valve replacement underwent significant changes in 2014, upon the growing success of TAVR.5 The ACC/AHA recommends TAVR in the following situations:
An approach by a heart valve team (consisting on experts in valve heart disease, interventional cardiology, cardiac imaging, cardiac anesthesia, and cardiac surgery) is essential in patients who are being considered for TAVR (class I indication, level of evidence C). The improvement in TAVR outcomes has been attributed to the selection of the right patients which is done by the heart valve team.12
With the evolution of minimally invasive percutaneous coronary interventions to treat complex coronary artery disease, cardiologists aimed to treat stenotic valves percutaneously with minimal risk. This journey began with the development of balloon aortic valvuloplasty.13 However, it failed to substantially change long-term outcomes. In 2002, Cribier and colleagues described the first human case of TAVR, in a 57-year-old man with severe calcified bicuspid AS.14 Webb and colleagues subsequently reported the feasibility and safety of TAVR via a transfemoral arterial approach.15 The first large randomized trial of TAVR was PARTNER trial in 2010. This trial reported that 1-year mortality was 24.2% for TAVR and 26.8% for surgery aortic valve replacement (P=0.001 for noninferiority).16 Over the past decade, approximately 15,000patients have been randomized in clinical trials of TAVR, and many other patients will be randomized in ongoing and future studies that intend to broad indications, compare devices, simplify the procedure, and optimize clinical outcomes.17
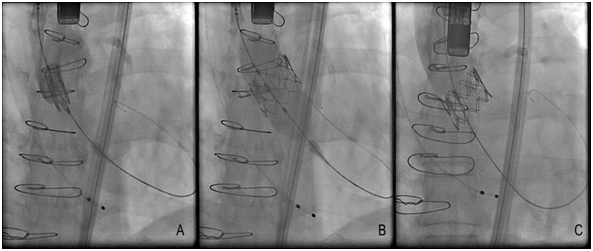
There are several approaches to transcatheter aortic valve replacement which include transfemoral, transapical, transaxillary, transubclavian, transcarotid, transcaval, and transaortic. The most common approach is transfemoral (Figure 1) and it appears to be safer in observational analyses than alternative access.18 An important determinant for transfemoral access is size of the iliofemoral circulation and its trajectory, as measured on a pre-operative CT scan. Patients with smaller iliofemoral vessels can develop more vascular complications.19 There is a trend, in selected cases and using transfemoral access, to perform the procedure without general anesthesia, intubation and surgery dissection. If patients do not have suitable vascular anatomy for transfemoral access, then an alternative approach can be undertaken in hybrid cardiovascular suites, which allow for integration of interventional cardiology and cardiac surgical techniques. The transfemoral vascular access for TAVR is the best choice for the procedure and improves the patient’s functional recovery.20 The size reduction of the delivery catheter of several TAVR systems has therefore increased the proportion of patients suitable for the transfemoral approach. For example, The Core Valve Evolutes R (23, 26, 29 mm; Medtronic, Dublin, Ireland) and SAPIEN 3 (23, 26 mm; Edwards Life sciences, Irvine, CA, USA) can be implanted using 14 Fr Inline sheath (Medtronic) or expandable-sheath technology, respectively. These simple systems can facilitate transfemoral TAVR in vessels as small as 5.5 mm (SAPIEN 3) or even 5.0mm (Evolut R).
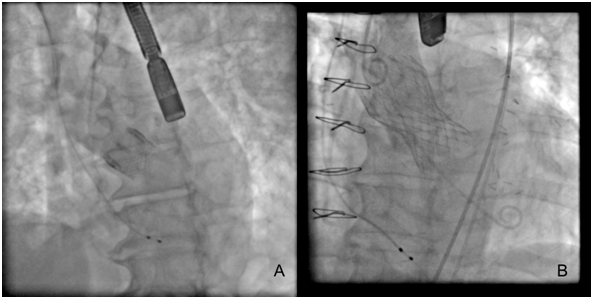
Until this moment, the TAVR devices approved and non-approved are described in (Table 1), and some TAVR devices have their fluoroscopic view demonstrated in (Figure 2). There was a recent report about Lotus valves recall due to pin troubles, following reports of problems with the device’s locking mechanism. The problem, according to the company, is the premature release of a pin connecting the Lotus valve to the device’s delivery system. Boston Scientific expects to reintroduce the Lotus to Europe and other regions in the fourth quarter of this year (2017).20 The most patients considered for TAVR undergo multimodal imaging assessment of the aortic root. The multislice computed tomography and/or three-dimensional echocardiography are the imaging modalities of choice (according to availability and local expertise) to evaluate the annular dimensions (diameters, area, perimeter) and unique anatomical features of each patient. A variety of adverse anatomical features may complicate TAVR procedure, and in such cases the availability of multiple TAVR technologies can facilitate the procedure safety.21
Approved TAVR Systems |
Non-Approved TAVR Systems |
Core Valve (Medtronic, Dublin, Ireland) |
HLT valve (Heart Leaflet Technologies Inc., Maple Grove, MN, USA) |
SAPIEN (Edwards Lifesciences, Irvine, CA, USA) |
Colibri valve (Colibri Heart Valve, LLC, Broomfield, CO, USA) |
SAPIEN XT (Edwards Lifesciences) |
Trinity valve (Transcatheter Technologies GmbH, Regensburg, Germany) |
JenaValve (JenaValve Technology GmbH, Munich, Germany) |
TRISKELE valve (University College London, London, UK) |
Symetis ACURATE (Symetis SA, Ecublens, Switzerland) |
Biovalve (Biotronik AG, Bülach, Switzerland) |
Core Valve Evolut (Medtronic) |
Inovare (Braile Biomedica, São José do Rio Preto, Brazil) |
Portico (St. Jude Medical, St. Paul, MN, USA) |
Micro Port (Micro Port Medical Group, Shanghai, China) |
Direct Flow Medical Aortic Valve (Direct Flow Medical Inc., Santa Rosa, CA, USA) |
|
Lotus (Boston Scientific, Marlborough, MA, USA) |
|
Medtronic Engager (Medtronic) |
|
SAPIEN 3 (Edwards Lifesciences) |
|
Core Valve Evolut R (Medtronic) |
|
Table 1 Describes the TAVR devices approved and non-approved systems.
There are specific complications associated with TAVR that include stroke, bleeding, paravalvular leak, and AV blocks requiring permanent pacing. When comparing the complications of TAVR vs. surgical AVR in High-risk patients, major stroke and high-grade AV block at 30 days was higher in the TAVR group.22 This increased stroke risk was no longer present at 5-years (10.4 vs. 11.3%, p=0.61).23 Newer technologies, such as cerebral protection devices, significantly reduced the number and size of brain lesions detected by magnetic resonance imaging following TAVR.24 Early TAVR experiences yielded a procedural success rate of 70% to 80%,2 in addition, the Society of Thoracic Surgeons/ACC Transcatheter Valve Therapy Registry investigators have reported a 92% procedural success rate since the approval of TAVR in the U.S.25,26 Therefore, TAVR have been a promising procedure and very tolerable.
The results of the published PARTNER 2trial, which compared TAVR outcomes (in this case outcomes of the SAPIEN XT valve system) with surgery AVR have shown similar overall outcomes with respect to primary endpoints (death and stroke) at 2years between the 2groups, although the results favored TAVR in patients who underwent TAVR through the transfemoral route.27 TAVR resulted in fewer bleeding sequelae, lower rates of acute kidney injury and new-onset atrial fibrillation, and also shorter hospitalization, intensive-care stay, and larger aortic valve areas when compared to surgery. But TAVR had a higher rate of paravalvular leak and vascular sequelae than surgery. The results of the Edwards SAPIEN 3 study included a reduced rate of vascular sequelae because of the smaller sheath size and a lower rate of paravalvular regurgitation because of the skirt mechanism.28
The Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial is the Medtronic’s Core Valve version of the PARTNER 2 trial. In SURTAVI, the 2-year rate of all-cause death or disabling stroke was 12.6% in patients who underwent TAVR with either the first-generation Core Valve prosthesis or next-generation Evolut R (both Medtronic) and 14.0% with surgery, this difference meets the criteria for no inferiority.29 The results of SURTAVI and PARTNER 2A support use of TAVR in some intermediate-risk patient. It is important to address the following questions before the TAVR procedure: whether a bioprosthetic valve is preferred over a mechanical valve, and whether the long-term durability of the valve is a concern for a particular patient.
In the meantime, the SAPIEN 3 prosthesis has been approved for use in intermediate surgical risk patients in the U.S. Moreover, the FDA has recently approved clinical trials to test the efficacy of TAVR in low-risk patients, both for the Medtronic arm using the Core Valve Evolut R System (NCT02701283) and the Edwards SAPIEN arm using the Edwards SAPIEN 3 valve system (PARTNER 3 trial, NCT02675114). This newer-generation valves have the potential to improve the success of the procedure and to reduce sequelae. The ability to reposition and recapture the Core Valve Evolut R valve has given Physicians confidence during the procedure. The Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of LOTUS Valve System Randomized Clinical Evaluation (REPRISE III) trial enrolled 1014patients and evaluated the safety and efficacy of the LOTUS Edge Valve (Boston Scientific Corporation; Natick, Mass) for TAVR. Finally, the Direct Flow Medical Transcatheter Aortic Valve System (Direct Flow Medical, Inc.; Santa Rosa, Calif), a part of the TranScatheter Aortic Valve Replacement System US Feasibility (SALUS) trial, was designed to minimize aortic regurgitation while replacing the faulty native aortic valve in high- and extremely high-surgical-risk patients.
Focusing on intermediate and low-risk patients, UK-TAVI (ISRCTN57819173) will include 808patients, randomized to TAVR in the United Kingdom with any commercially available TAVR device vs. surgery AVR. The primary endpoint is all-cause death at one year with additional cost-effectiveness analyses. Two other non-inferiority trials using balloon-expandable (PARTNER 3, NCT02675114) and self-expanding prostheses (Evolut R Low Risk, NCT02701283) have initiated the recruitment of patients with predicted perioperative mortality<2% and <3%, respectively. PARTNER 3 randomizes transfemoral TAVR with the SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) vs. surgery AVR with a bioprosthetic valve, and compares a primary composite endpoint at one year including all-cause mortality, all strokes, and rehospitalization. The Evolut R Low Risk trial randomizes TAVR with the Evolut R and Core Valve prostheses compared vs. surgery AVR with a bioprosthetic valve, with a primary composite endpoint at two years that included all-cause mortality or disabling stroke. Therefore, is necessary to wait for further researches for this treatment of patient with severe AS and some questions need answers, like the durability of transcatheter valves may be shorter than that of surgical bioprostheses. The longest available clinical follow-up concerning a substantial number of TAVI patients is limited to five years, in which excellent valve performance has been demonstrated.30,31 However, as with surgical bioprostheses, transcatheter aortic valves are likely to degenerate with time and may eventually require repeat intervention.32
TAVR is an effective procedure, in which a bioprosthetic valve is implanted within the severely stenotic native aortic valve. It is a new therapeutic modality for treatment of patients with severe symptomatic aortic stenosis and other comorbidities, who have inherently a high surgical risk. Some multicenter registry and randomized data have suggested non-inferiority of TAVR in intermediate-risk populations when compared with surgery AVR; however, extrapolation of trial results to younger age groups is problematic, particularly given the lack of long-term durability data. For patients with a high operative risk or those older than 75years, decisions regarding the most appropriate treatment strategy should be undertaken by the Cardiology Team following a careful review of the risks and benefits of each approach. By increasing the technical expertise, reducing complications related to the procedure and improving technological advances and delivery systems, the use of this technique to treat patients with severe AS but without significant comorbidity, who now undergo open-heart surgery, may become possible in the future. Upcoming TAVI studies are expected to address knowledge gaps while expanding current indications and refining clinical outcomes both acutely and during long-term follow-up.
The authors would like to express their gratitude to the staff of the international clinical pathology journal for the invitation to make this mini review.
The author declares no conflict of interest.

©2017 Vilela, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.