eISSN: 2471-0016


Case Report Volume 6 Issue 2
1Department of pathology, India
1Department of pathology, India
Correspondence: Tazeen Jeelani, senior resident department of pathology SKIMS soura Srinagar Jammu and Kashmir, India, Tel +9190 7012 9836
Received: January 01, 1971 | Published: April 27, 2018
Citation: Jeelani T, Mushtaq S, Makhdoomi R. Primary pulmonary MPNST?A rare case report. Int Clin Pathol J. 2018;6(2):121–122. DOI: 10.15406/icpjl.2018.06.00170
Primary lung sarcoma is an extremely rare tumour accounting for <0.5% of all the lung tumours. Here we present a case of primary pulmonary malignant peripheral nerve sheath tumour (MPNST) in a 60 year male patient. The diagnosis was made on histopathological examination (HPE) and confirmed on immunohistochemistry (IHC). Patient underwent surgery and received 2 cycles of post‒operative chemotherapy however, expired eight months after diagnosis. We conclude that MPNST should be included in the differential diagnosis of spindle cell tumours of lung.
Keywords: MPNST, IHC, lung cancer, sarcoma, primary
MPNST is one of the rare tumours. Its incidence is 1 per 1,000,000 and represents about 3% to 10% of all soft tissue sarcomas.1 MPNST can be seen in any part of the body like head and neck, extremities, trunk or retroperitoneum,2,3 but intra‒thoracic primary pulmonary MPNST with or without NF‒1 is uncommon. Only few adult cases have been reported so far.4,5
A 60 years old smoker, normotensive, non‒diabetic presented with chief complaints of cough, fever and weight loss for 20 days. On examination the patient was conscious, co‒operative, well oriented, with a respiratory rate of 18/min, blood pressure of 120/70 and pulse rate of 86/min. Cardiovascular and per‒abdomen examination were clinically normal. However, on auscultation decreased breath sounds were found on left lower side of chest. Chest radiograph revealed a lesion in the left lung. CT scan (contrast enhanced) of chest was done which showed a 71x59mm cystic lesion in the superior segment of left lower lobe. Cyst showed internal septations without calcification. USG abdomen and PFT (pulmonary function tests) were normal. Hydrated serology was negative and not suggestive. Routine complete blood counts, LFT (liver function test) and KFT (kidney function tests), were within normal limits. The patient underwent left postero‒lateral thoracotomy with lower lobe lobectomy. Intra‒operatively there was large bronchogenic mass occupying almost whole of the left lobe with multiple hilar nodes. On gross examination, we received a lobe of lung measuring 15x9.5x5cm. Serial slicing of the lung showed a well circumscribed mass measuring 7x8cm. Cut section showed variegated appearance with extensive hemorrhagic and necrotic areas. On light microscopy a spindle cell tumour was seen with cells having moderate to severe pleomorphism and mitosis of >10/10hpf, and extensive areas of necrosis (Figure 1a) (Figure 1b).
Impression of grade 3 sarcoma (T.D=3, mitosis=2, necrosis=2) was made based on FNCLCC grading system. Diagnostic possibilities considered were:
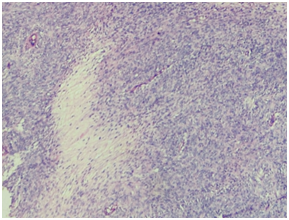
Figure 1a Tumour showing areas of geographical necrosis with surrounding tumour cells at low power magnification.
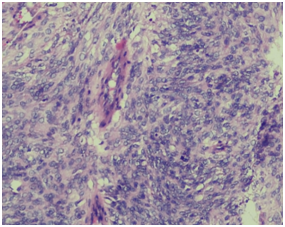
Figure 1b Higher magnification showing highly pleomorphic tumour cells (epitheloid type) arranged vessel with few mitotic figures.
IHC was done, tumour cells showed positivity for S‒100 (Figure 2), and negative for desmin, CD34, HMB‒45, CD99 and CK which was consistent with the diagnosis of MPNST. The patient received two cycles of post‒operative chemotherapy (Ifosfamide+adriamycin) but expired eight months after diagnosis.
Primary pulmonary sarcomas (PS) comprise less than 1% of primary lung tumours. They represent 40% of rare pulmonary tumours and about 9% of all soft tissue sarcomas.6 MPNST is one of the rare soft tissue tumours comprising about 5‒10% of all soft tissue sarcomas.7,8 These tumours are difficult and often create problem in diagnosis because of their histological similarities with other spindle cell sarcomas. Histological mimics include; leiomyosarcoma, monophasic synovial sarcoma, and fibro sarcoma.9 MPNST can arise from branches of major or minor peripheral nerves or their sheath.10 They can arise either spontaneously, or may have an association with neurofibromatosis type‒1 (NF‒1) in about 5‒42% of adult cases. Although aggressive in nature, still surgery remains mainstay of treatment for MPNST lung.9
It has been reported that, 5‒year survival rate may vary from 15‒40%. In patients where disease is associated with NF‒1 prognosis is generally poor. Associated adverse prognostic indicators include tumour size >5cm, mitotic rate >20/10hpf, central location of tumour and incomplete resection.11 For diagnosing this tumour combination of gross, tumour histology and immunohistochemistry should be used.9 Immunohistochemically tumour cells show reactivity for S‒100 protein and Leu‒7, in approximately half of the cases.12 S‒100 tends to be focal and not particularly strong, except in epitheloid variant of this tumour.13 Differential diagnosis of primary pulmonary MPNST include metastasis of an extra thoracic mesenchymal tumour into lung. The distinction of pulmonary MPNST from other primary lung sarcomas especially malignant mesenchymal tumours (like synovial sarcoma and leiomyosarcoma) is very difficult but crucial as the treatment options and prognosis differ.14
None.
Authors declare there is no conflict of interest.

©2018 Jeelani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.