eISSN: 2471-0016


Case Report Volume 4 Issue 4
1Department of Pathology, Swami Vivekananda Subharti University Merrut, India
1Department of Pathology, Swami Vivekananda Subharti University Merrut, India
2Department of Pathology, Manipal University, India
2Department of Pathology, Manipal University, India
Correspondence: Mamta Gupta, Associate Professor, Department of Pathology, Subharti Medical College, Swami Vivekananda Subharti University, Subhartipuram.NH58, Meerut250005 , Uttar Pradesh, Tel 9195 5754 2520
Received: January 01, 1971 | Published: April 24, 2017
Citation: Gupta M, Lobo FD, Adiga D. Fine needle aspiration cytology of sacrococcygeal chordoma- utility in a case of clinical dilemma. Int Clin Pathol J. 2017;4(4):88–90. DOI: 10.15406/icpjl.2017.04.00101
Chordoma is a rare malignant tumor that arises from remnants of the fetal notochord affecting the axial skeleton. We report an unusual case of sacrococcygeal chordoma, clinically showing a close resemblance to pilonidal sinus. On fine needle aspiration cytology of the lesion, tumour showed varied but characteristic diagnostic morphological features.
Keywords: cytology, chordoma, sacrococcygeal, physaliphorous cells
Chordoma was originally described by Virchow in 1857 and further characterized by Ribbert in 1894. It is a rare low to intermediate grade malignant notochordal tumor that recapitulates the notochord and has a tendency for recurrences and metastasis.1,2 They represent 1–4% of all malignant bone tumors. Most prevailing theory regarding the development of chordoma is that the notochord fails to degenerate and undergoes malignant transformation. Approximately 50% of chordomas are sacrococcygeal in origin and usually present as destructive bone lesions with a large soft tissue mass.3–5
FNAB is a safe, simple and quick method of early pre- operative diagnosis of chordoma. However, because of various overlapping cytologic features between chordoma, chondrosarcoma and metastatic clear cell carcinoma, it is important to recognize the various appearances of chordoma in FNAB.6,7 The cytologic features, combined with classic radiologic and clinical presentations, allow for correct cytologic diagnoses to be established in most case.8 The case is presented because of its rare presentation, role of cytopathology in the early pre-operative diagnosis and differentiation from other entities.
A 54 year-old male presented to the surgical out-patient department. He complained of lower backache and swelling in the gluteal region since many years. The swelling had gradually progressed to the present size. There was a recent rapid increase in size of swelling, accompanied by formation of a discharging sinus of three months duration. On examination, a 5 X 4 cm mass was present in the intergluteal fold, the overlying skin showed a discharging sinus measuring 1 X 1 cm (Figure 1). Clinical diagnosis of Pilonidal sinus was made. Routine investigations were within normal limits. The patient was referred for fine needle aspiration cytology. The smears showed abundant myxoid stroma with singly dispersed small, round cells with bland nucleus along with cells having abundant vacuolated bubbly cytoplasm and small nucleus (Figure 2). A diagnosis of myxoid rich soft tissue tumour with possibility of chordoma was suggested. Lumbar magnetic resonance imaging done subsequently revealed a well demarcated, multilobulated mass in the sacococcygeal region (Figure 3).

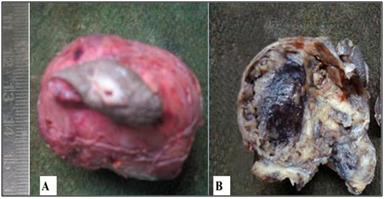
The mass was excised and sent for histopathological examination. Gross examination showed a circumscribed soft tissue mass covered with an elliptical piece of skin with ulceration and sinus formation. Cut surface was firm to hard, vaguely lobulated and variegated with reddish brown hemorrahagic to gelatinous areas (Figure 4). Histopathology revealed a tumour composed of cells arranged in lobulated pattern separated by fibrous septa. The stroma showed myxoid areas and areas of hemorrhage and necrosis. The tumour cells were polyhedral to round with eosinophilic cytoplasm and round atypical nuclei along with physaliphorous cells. Occassional spindle shaped stellate cells and signet ring-like cells were also seen. Physaliphorous cells were large multivacoulated with bubbly appearance of cytoplasm and small inconspicuous nuclei (Figure 5). The cytoplasmic vacuoles were periodic acid Schiff (PAS) positive and diastase sensitive suggesting glycogen deposition (Figure 6). The tumour cells were seen infiltrating the capsule and surrounding soft tissue. The skin overlying tumour showed ulceration and chronic inflammatory cell infiltrate in the dermis. Diagnosis of sacrococcygeal chordoma- NOS was made.
The indolent nature and unpredictable behaviour of sacrococcygeal chordomas make early detection difficult. By the time the diagnosis is established, the tumour is usually very large as in the present case. Local invasiveness and destructiveness are characteristic features of the disease.5 Chordoma most commonly presents between the fifth and seventh decades. The clinical presentation is related to the location and spread of the neoplasm. The axial skeleton, commonly, the sacral region, followed by the spine and base of skull are well-documented sites of occurrence. In sacrococcygeal presentation, pain is the most common symptom referred to the tip of spinal column or lower back pain.2,3
Rarely, because of slow growth, long standing non specific symptoms and location the lesion can be mistaken for a pilonidal cyst.1 or a pinonidal sinus.9 The present case closely mimicked a pilonidal sinus clinically. Complete surgical excision is the only therapeutic modality able to affect a cure. Imaging techniques, in particular contrast resolution afforded by MRI, play a crucial role in surgical planning.5
The WHO histological subtypes of chordoma include chordoma–NOS, chondroid chordoma and dedifferentiated chordoma.3 Microscopic differentiation of these variants is important as chondroid chordoma has a favorable prognosis and dedifferentiated chordoma characterized by high grade spindle cell component in addition to conventional histology has a poor prognosis.2,3 Presence of classic physaliferous cells containing centrally located nucleus, scalloped by multiple cytoplasmic vacuoles embedded in myxoid to chondromyoid stroma, on fine needle aspiration is an essential for the diagnosis of chordoma. However, such classic cells are rare.8,10 These cells develop from stellate or primordial cells, which proceed through intermediate cells, vacuolization stage and then progress to destruction.4In dedifferentiated chordoma anaplastic spindle cells with small amount of myxoid matrix and rare or absent physaliferous cells are seen.11
The microscopic differential diagnoses of chordoma include extraskeletal myxoid chondrosarcoma, chondrosarcoma, myxopapillary ependymoma, liposarcoma, metastatic mucinous adenocarcinoma, and metastatic renal cell carcinoma.2 Chondrosarcomas demonstrate cartilaginous differentiation with an abundance of often myxoid matrix, cytologically indistinguishable from chordoma. However, vacuolated chondrosarcoma cells have a perinuclear halo instead of the bubbly cytoplasm of physaliferous cells. Distinction between chordoma and mucinous adenocarcinoma may be difficult due to the presence of signet ring cells that mimic ‘‘chordoma cells’’ and needs careful evaluation of their nuclear-cytoplasmic features. FNAC of myxoid liposarcoma may be differentiated from chordoma by its plexiform capillary network and presence of lipoblasts. The myxoid stroma of myxopapillary ependymoma is similar to chordoma, but its stroma is usually surrounded by clusters of cuboidal epithelium-like cells that are not seen in chordoma.8,11 Because of various overlapping cytologic features in these myxoid, chondromyxoid and mucoid rich tumours, it is important to recognize the subtle microscopic features which will enable the cytologist to reach a conclusive diagnosis on fine needle aspiration cytology. An ultrastructural and histochemical study shows that these vacuoles result from breakdown and utilization of membrane-bound glycogen in sulfated glycosaminoglycans biosynthesis. Immunohistochemically, physaliphorous cells express S100, cytokeratin and epithelial membrane antigen.2,4
Prognosis has considerably improved with newer surgical techniques of resection However, relentless local invasion of clinically sensitive regions results in a poor long term prognosis.3 The present case of sacrococcygeal chordoma clinically closely mimicked a pilonidal sinus based on characteristic location and clinical presentation. FNAC provided an early pre-operative diagnosis of chordoma because of the distinct cytological features. Thus, emphasizing the importance of FNAC as an early diagnositic tool.
None.
The author declares no conflict of interest.

©2017 Gupta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.