eISSN: 2471-0016


Research Article Volume 1 Issue 4
1Department of Clinical Pathology, Brawijaya University, Indonesia
1Department of Clinical Pathology, Brawijaya University, Indonesia
Correspondence: Hani Susianti, Department of Clinical Pathology, Faculty of Medicine, Brawijaya University, Malang, Indonesia, Tel 6281 3347 60295
Received: January 01, 1971 | Published: December 9, 2015
Citation: Susianti H, Lie S, Yoavita. auto identify discrepancies between urine test strip and sediment results using cross check function on fully automated urine analyzer. Int Clin Pathol J. 2015;1(4):87–93. DOI: 10.15406/icpjl.2015.01.00020
Background: Urine chemistry (test strip/dipstick) and microscopy examination are the basic procedure of urinalysis. UX-2000 is the fully automated urine analyzer from Sysmex Corporation (Japan) which integrate urine test strip and urine particle assay on one platform. This instrument is also equipped with crosscheck function which can correlate the test strip and urine particle results and the flag for discrepancies.
Aim: The aim of this study was to evaluate the crosscheck function of UX-2000 and to assess its impact on laboratory efficiency.
Methods: 250 urine samples were collected and analyzed using UX-2000. Crosscheck results were obtained on 4 combination parameters: blood (BLD) & red blood cell (RBC), leukocyte esterase (LEU) & white blood cell (WBC), protein (PRO) & cast (CAST), and nitrite (NIT) & bacteria (BACT). Impact of crosscheck function on laboratory efficiency is determined using turn around time evaluation.
Results: Crosscheck function results showed more than 85% concordance between urine test strip and urine sediment and raise flag for discrepancies. The application of this feature was able to reduce turnaround time from 7.5 into 5.4minutes.
Conclusion: The crosscheck function of UX-2000 enabled more efficiency to identify discrepant results and reduce turnaround time urinalysis examination.
Keywords: fully automated urinalysis, urine test strip, urine sediment, crosscheck function
BLD, blood; RBC, red blood cell; LEU, leukocyte esterase; WBC, white blood cell; PRO, protein; CAST, cast; NIT, nitrite; BACT, bacteria
Urinalysis is important for establishing early diagnosis, evaluating the disease severity, and monitoring of various kidney and urinary tract disorders.1 These evaluations typically performed by assessing the physical and chemical characteristics of urine using urine test strip and analyzing urine sediment through manual microscopy.2,3 However, manual urine test strip analysis have been reported to have low sensitivity due to many interfering factors that can induce false-negative or false-positive reactions. Its use also relies on the subjective interpretation of the colour change intensities, which can give inconsistent results.4,5 Likewise, manual microscopy is also time-consuming, labor-intensive, and has a wide interobserver variability.6,7
Moreover, test strip results often different from sediment findings, creating confusion among clinicians when interpreting the urinalysis results. Discrepancies between blood (BLD) & red blood cell (RBC), leukocyte esterase (LEU) & white blood cell (WBC), nitrite (NIT) & bacteria (BACT), and protein (PRO) & cast (CAST) parameters are the most frequent observation owing to different limitations of urine test strip and microscopic examination.8 As urinalysis being one of the most commonly carried out routine examination in clinical laboratories, individual evaluation for each discrepant results will not only require a substantial time, but also require a significant number of laboratory technicians. This can definitely cause problems in laboratories with limited technicians.
Many attempts are continuously made to overcome those limitations.9 The development of automated urine analyzers based on flowcytometry during the past few years have allowed faster, accurate, and more standardized cells and particles counting. Several studies reported that this technology is good to be used in urinalysis.10,11 The combination of urine test strip and flowcytometry results have also been reported with better sensitivity so leading to a significant reduction in manual microscopy rate.12,13 Recently, UX-2000 (Sysmex Corporation, Japan), a fully automated analyzer which enable to analyze physical, chemical and sediment characteristics of urine sample on a single platform, has been introduced. This instrument does not only integrate urine test strip and flowcytometry assay, but also equipped with crosscheck function to correlate the results obtained from both methods.14,15 This device is expected to further improve the efficiency and quality of urinalysis and workflow of laboratories. The aim of this study was to evaluate the crosscheck function of UX-2000 and to assess its impact on the efficiency of laboratory.
Samples
This study was conducted in the Central Laboratory of Dr. Saiful Anwar General Hospital Malang, East Java, Indonesia. There were 250urine samples included in the study were tested within the period of September to December 2014. Since vitamin C is a strong reducing agent that can produce false-negative glucose, blood, and nitrite test strip results, subjects on vitamin C supplementation were excluded from this study. Samples with volume less than 15mL and/or stored more than 2hours post-collection were also excluded. Informed consent was obtained from all participants.
Urine collection procedure
Urine samples were obtained by mid-stream voiding technique using a sterile, transparent, disposable and non-preservative container. All samples were analyzed within 2hours after collection using UX-2000. Manual microscopy would be performed if there were discrepancies between test strip and sediment results.
UX-2000 and crosscheck function analysis
UX-2000 is a fully automated urine analyzer that integrates physical, chemical (urine test strip), and sediment (flowcytometry) analysis. It requires at least 5mL of urine sample (aspirated volume 2.2mL). This device comprises two analytical components, namely chemical (CHM) and flowcytometry (FCM) units. CHM and FCM analysis can be reported as a merged or individual results.14 The CHM unit analyzes the physical and chemical characteristics of urine samples. It utilizes three principles for physical analysis of urine, which are transmission refractometry method for specific gravity analysis, reflectivity measurement method for color hue analysis, and light-scattering measurement method for turbidity analysis. Chemical analysis are performed using dual-wavelength reflectance method for glucose (GLU), protein (PRO), bilirubin (BIL), urobilinogen (URO), pH (pH), ketone bodies (KET), nitrite (NIT), and leukocyte esterase (LEU) and single wavelength reflectance method for blood (BLD). The results are displayed in qualitative or semiquantitative values with a parallel rank number.14
FCM unit uses flowcytometry technology to count and analyze microscopic particles present in urine. After staining with specific fluorescent polymethine dye, laser light is used to generate forward scattered light signal, lateral scattered light signal and the fluorescent light signal from each urine particle. These signals are converted into electrical signals, which are detected and then each particle is categorized as red blood cell (RBC), white blood cell (WBC), epithelial cell (EC), cast (CAST), or bacteria (BACT). The results are presented in quantitative units and are also displayed as the histogram, and scattergrams.14 Quality assurance was implemented using accuracy and precision tests. Quality control was performed using Meditape Check 1 & 2 for CHM unit and two levels (high and low) of UF II Control material for FCM unit. We interpreted a coefficient of variation (CV) of less than 20% as good performance for between-run imprecision assessment. This value was chosen based on data reported by studies on instrument with similar function.12,16,17
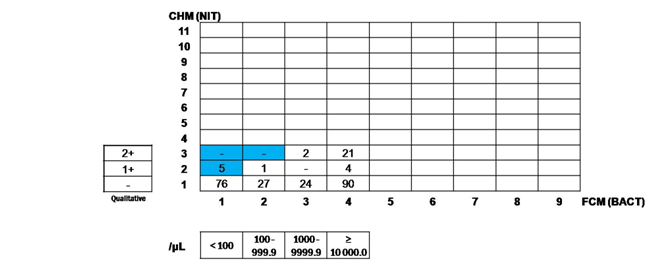
When the on-board crosscheck function is activated, certain related parameters of CHM and FCM units will be compared. A “crosscheck flag” is displayed if the results of those parameters show discrepancies. The cut-off values to trigger the crosscheck flag (rank values) are already set by the manufacturer, but user can make modifications in accordance with the laboratory needs. Optimal cut-off value for each user modified rank is set according to the reference values that suit the laboratory’s specific population. The greater the rank’s number, the higher the cut-off value. Samples with discrepant results should undergo further confirmation by manual microscopy. An example of crosscheck function application can be seen in Figure 1. If BLD result is 2+ (set as rank 7) and RBC result is 383.9/μl (set as rank 4), we will find that the intersecting line will be located at the white coloured cell. It means that there is a concordance between BLD and RBC. On the contrary, if we find a case which the intersecting line will be located at the blue coloured cell, it means that there is a discrepancy between BLD and RBC and UX-2000 will judge a discrepancy and give “crosscheck” warning. Blue and white colour are arbitary. Rank values applied in this study were developed according to the reference values that were used in our laboratory.
Manual microscopy
Manual microscopy was performed according to the standard procedure used in the laboratory (based on NCCLS guideline). After 10mL of each urine sample was centrifuged at 2000rpm (400g) for 5min, 9.5mL of supernatant was discarded. The remaining urine samples were resuspended and 50μL of urine was pipetted onto a glass slide, covered with a coverslip (22mm×22mm), and examined under a CX21 light microscope (Olympus, Japan). A minimum of 10 fields at 400×magnification (high power field, HPF) for cells or crystals and at 100× magnification (low power field, LPF) for casts were examined.18 The counts were given as an average per HPF or LPF, depending on the types of particles. To minimize interobserver variability, all manual microscopic examination was performed by one qualified laboratory technician.
Statistical analysis
Data was analyzed using MS Excel 2007(Microsoft, USA). Assessment of crosscheck function was done by determining the concordance and discordance level between BLD & RBC, LEU & WBC, NIT & BACT, and PRO & CAST. Concordance is defined as the degree of agreement between two measuring or rating techniques. Concordance is calculated as follows:

In this study, the term disconcordance (“significant discrepancy”) was considered if there were a difference of more than 1 rank between CHM qualitative results and corresponding obtained FCM results converted into rank values.19,20 Positive concordant samples refer to all samples with concordant results between CHM and FCM analysis, whereas negative concordant samples refer to all samples with discordant results between both analyses.
Performance evaluation of UX -2000
The between-run imprecision data of UX-2000 in our laboratory are presented in Table 1 & Table 2. The imprecision for BLD, LEU, PROT, NIT, RBC, WBC, EC, CAST and BACT were lower than 20%. The imprecision for EC and CAST at low cell concentration was higher than the other parameters on UX-2000.
Parameters |
Meditape Check I |
Meditape Check Ii |
||
|---|---|---|---|---|
Mean Value |
CV (%) |
Mean Value |
CV (%) |
|
BLD |
88.5 |
0.51 |
14.7 |
2.48 |
LEU |
60.9 |
1.32 |
94.6 |
0.16 |
PROT |
73.4 |
0.48 |
39.3 |
1.29 |
NIT |
99.3 |
0.31 |
68.6 |
0.64 |
Table 1 Between-run imprecision of UX -2000 measured with Meditape Check I and Meditape Check II for CHM unit.
Parameters |
High Level |
Low Level |
||
|---|---|---|---|---|
Mean Value |
CV (%) |
Mean Value |
CV (%) |
|
RBC |
176.0 |
4.87 |
36.7 |
8.89 |
WBC |
785.3 |
2.75 |
40.7 |
3.80 |
EC |
64.9 |
4.82 |
9.7 |
16.19 |
CAST |
18.3 |
9.65 |
4.5 |
17.43 |
BACT |
704.7 |
2.94 |
180.9 |
9.68 |
Table 2 Between-run imprecision of UX -2000 measured with UF II Control for urine sediment (FCM unit).

Concordance between CHM and FCM results
In this study, 250 urine samples were analyzed.21 Of these, 214 (85.6%) samples showed an agreement (concordance) between BLD and RBC results (Table 3) and 229 (91.6%) samples showed an agreement between LEU and WBC results (Table 4). Concordance were also seen in 245 (98%) samples for NIT and BACT (Table 5) and in 226 (90.4%) samples for PRO and CAST (Table 6). Samples giving discordant results will subjected to manual microscopic examination. Thirty-six (15%) samples gave discrepant results between BLD and RBC, with 25 (69.4%) samples showed RBC rank that was higher than the corresponding BLD rank and 11 (30,6%) samples showed BLD rank that was higher than the RBC rank. Discrepancies between LEU and WBC were shown in 21 (8,4%) samples, with 14 (66,67%) samples had higher WBC rank and 7 (33,33%) samples had higher WBC rank (Table 7). Between NIT and BACT, discrepant results were given by 5 (2%) samples, all of which had NIT rank higher than the corresponding BACT rank. Discrepancies between PRO and CAST were seen in 24 (9,6%) samples, with all samples showing CAST rank that was higher than the corresponding PRO rank. All samples with discrepant results sent for microscopic confirmation, and the concordance rate of BACT, WBC, CAST and RBC were 100%, 85.7%, 70.8% and 50%, respectively.


Parameters |
Concordance |
Discrepancy |
||
|---|---|---|---|---|
n |
% |
n |
% |
|
BLD & RBC |
214 |
85.6 |
36 |
14.4 |
LE & WBC |
229 |
91.6 |
21 |
8.4 |
NIT & BACT |
245 |
98.0 |
5 |
2.0 |
PRO & CAST |
226 |
90.4 |
24 |
9.6 |
Table 7 Concordance and discrepancy results between BLD & RBC, LEU & WBC, NIT & BACT, and
PRO & CAST.
Turnaround time
Before Implementing UX-2000, our laboratory was still applicated unintegrated urinalysis testing. For urine test strip (physical and chemical analysis) procedure, we used Siemens Clinitek Advantus (Siemens Healthcare GMBH, Germany), a semiautomated urine test strip analyzer and for urine sediment procedure, we used Sysmex UF-500i (Sysmex Corporation, Japan). By using UX-2000 (integrated urine analyzer) and activating its crosscheck function, we identify that our Turn around time (TAT) mean significantly reduced from 7.5 into 5.4 minutes. In this study, we defined TAT as the time from confirmed arrival of each specimen up to the reporting of its analysis results. The mean TAT of specimens by different methods was used for the comparison.19
Urinalysis is one of the most common laboratory test which need to be readily available in any healthcare system and non invasive collected specimen. There are three steps in performing a complete urinalysis: physical characteristic, chemical characteristic, and microscopic examination. It is often observed that test strips and microscopy testing results show discrepancy and poses challenge to the laboratory for reporting and to clinicians, for interpreting the results. The discrepancy in urinalysis refers to pathological, interference, or certain conditions. Nevertheless, urine test strip and sediment testing are two related laboratory procedures that are complementary and cannot substitute each other. Thus, evaluation regarding the concordance level and discordance results between these two is required. In this study, between-run imprecision of UX-2000 was good for BLD, LEU, PROT, NIT, RBC, WBC, EC, CAST, and BACT based on CVs less than 20%. However, at low cell concentration, EC and CAST showed higher CVs than the rest of the FCM unit parameters. This data were similar to those reported for the Sysmex UF-1000i, an automated urine sediment analyzer with similar principles to that of the FCM unit of UX-2000. Higher CVs of EC and CAST are probably related to the variation in the morphology of EC and CAST, which make their identification difficult.12
The agreement of urinalysis results between CHM and FCM analyzes was good, as indicated by the concordance level of more than 85% for each pair of parameters compared. However, these results were different than those reported by Miyazaki,19 which were 85.6% vs 93.3%, 91.6% vs 96.1%, 98% vs 100%, and 90.4% vs 93.4%, for BLD and RBC, LEU and WBC, NIT and BACT, and PRO and CAST, respectively. Discrepancies between BLD and RBC as well as LEU and WBC in this study were similar with those reported reported by Miura et al.,20 which were 14.4% vs 11.9% and 8.4% vs 7.2%, respectively.20 Out of the 36 samples with discrepant results between BLD and RBC, 25(69.4%) samples showed RBC rank which was higher than the corresponding BLD rank, 18(72%) may be caused by the presence of yeast. Yeast can sometimes be falsely recognized as red blood cell by the instrument. Discrepancies in the remaining 7(28%) samples could be caused by the presence of oxidizing drugs or ascorbic acid in large quantities. On the contrary, higher BLD rank than the corresponding RBC rank in 11samples could occur with the presence of hemoglobin or myoglobin, mixture of oxides, and bacterial peroxidases. Lower rates of agreement between BLD and RBC in this study, when compared to Miyazaki’s results, may be attributed to the different RBC classification applied in determining the concordance rate. We classified RBC as 1-3cells/HPF for rank 2 and 4-9cells/HPF for rank 3, whereas Miyazaki classified RBC as 1-4cells/HPF for rank 2 and 5-9cells/HPF for rank 3. This classification was developed according to the reference value used in our laboratory.
The causes of higher WBC rank than the corresponding LEU rank were hypertonic urine, the presence of protein or glucose in high concentration, antibiotics use, and false recognition by the instrument due to small epidermal cells, small cytosolic inclusion cells, trichomonas, or bare nuclei of flat epithelium cells. In this study, out of the 14 samples that showed higher WBC rank than the corresponding LEU rank, 10 samples (71.4%) had high protein or glucose concentration and 4 samples (28.6 %) came from subjects who had a history of antibiotic treatment, such as Cefalexin and Gentamycin. Higher LEU rank than the corresponding WBC rank could occur as a result of alkaline/hypotonic urine or antibiotics use.22 Three samples were taken from subjects treated with Imipenem or Meropenem, whereas the causes in the remaining 4 samples were not clear. High agreement between NIT and BACT was probably related to the fact that the most common urinary tract infection (UTI)-causing microorganisms are gram-negative bacteria, which have the ability to convert nitrate into nitrite. Since not all bacteria are capable of this conversion, few discrepancies may be observed. Staphylococci, Enterococci, and Pseudomonas are some examples of the types of bacteria that are incapable of reducing nitrate.22 Although bilirubin can cause false positive nitrite tests, the cause of discrepancies between NIT and BACT in this study was not clear because bilirubin was not present in these samples.
All of samples with discrepant results between PRO and CAST in this study had CAST rank that were higher than the corresponding PRO rank. This discrepancies may be caused by the lack of sensitivity of the urine test strip or Jiang et al.12 reported that the diagnostic performance of Sysmex UF-1000i was still limited for the sensitivity and specificity of cast (69.81 and 85.80%, respectively). UX-2000 also cannot distinguish the types of pathological casts, so positive results of pathological casts would require microscopic confirmation. The implementation of UX-2000 in our laboratory was able to reduce the urinalysis TAT (Turn Around Time). This reduction was achieved through the contribution of two major factors. First, the laboratory technicians did not need to spend time anymore for checking discrepancy of every sample and every related parameter because the flag was raised by crosscheck function automatically. Second, with the integration of physical, chemical and sediment analysis, urine samples only need to be prepared once which will make the procedure faster. The available spare time can be used by the laboratory technicians to analyze the discrepant samples more thoroughly. The advantage of the UX-2000 is that one analyzer can carry out both CHM and FCM and the results of both analyses can be viewed on the same screen, which makes data management easier. Moreover, the cut-off values of the related parameters to raise a crosscheck flag, can be set based on the laboratory need. This feature can produce more reliable results by providing laboratory-specific reference value-based analysis.23 From the study results, we observed that the application of automated crosscheck function in routine urinalysis workflow did offer some advantages. If the result between CHM and FCM analysis were concordant, it increased the confidence level of examination because both the test strip and urine particle analysis gave accurate results. If the result appeared to be discrepant or disconcordant, crosscheck function provided a “warning” sign and the microscopic confirmation was then performed, thus minimizing the possibility of an abnormal sample to go unnoticed. The crosscheck function also allowed flexible rule-setting, so that each laboratory could set up their own reference value for CHM and FCM ranks. Jiang et al.12 evaluated four most widely used strategies for urinalysis. In their study, neither Clinitek Atlas, an automated urine test strip analyzer, nor UF-1000i alone was sufficient as a screening tool for urinalysis, regarding the sensitivity and microscopic review rate. A combination of the two methods showed significantly increased sensitivity for urine screening. Some scientists also suggest that the combination of automated urine test strip reader and urine flow cytometer may be a better choice for urine sample screening.12,22,24
UX-2000 crosscheck function not only provides a faster and more efficiency way to identify discrepant results of urinalysis, but also improve the reliability of analysis results through integrated data management and human error reduction.
The authors would like to express gratitude to the Director of Dr. Saiful Anwar General Hospital, Malang, the Dean of Faculty of Medicine, Brawijaya University, Malang, PT Sysmex Indonesia and Dr Sonu Bhatnagar for their support in the research process, technical support and the final editing of this manuscript.
All procedures were followed by the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all participants for being included in the study.
The author declares no conflict of interest.

©2015 Susianti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.