eISSN: 2373-6372


Clinical Paper Volume 14 Issue 3
Department of Physics, Science Schools,Yazd University, Iran
Correspondence: Shafaei Mohammad Ali, Department of Physics, Science Schools,Yazd University,Yazd, Iran
Received: May 08, 2023 | Published: June 28, 2023
Citation: Ali SM, Sadeghei N. Using TLD in determining the absorbed dose in the area outside the irradiation region after mastectomy. Gastroenterol Hepatol Open Access. 2023;14(3):82-87. DOI: 10.15406/ghoa.2023.14.00551
Introduction: The most common cancer in women worldwide is breast cancer and radiotherapy is the best treatment choice after mastectomy.1,2 In the radiation therapy after mastectomy, depending on the type of tumor, the area of the breast removed goes under radiotherapy.
Methods: TLD (Thermoluminescence Dosimetry) chips were used in this project. Typically, each patient under an irradiation of 200 cGy during 25 sessions received a total of 5000 cGy. Radiation therapies were performed in such a way that the patient was subjected to 10 MV photovoltaic radiations in a 10×10 Cm2 area in the mastectomy region. Meanwhile, according to the depth of the tumor inside the chest, the radiation of gamma rays of cobalt 60 was used in surrounding regions. The absorption dose coefficients were the same in the TLD obtained from the photovoltaic radiation 10MV and the gamma beam of cobalt 60.
Results: Then the absorbed dose as calculated by hospital dosimeters standard was compared with the absorbed dose measured by TLD. The areas determined as zero absorption doses by Hospital dosimeters standard were calculated as non-zero absorbed dose by TLD. There were also areas where the doses calculated by TLD were different from the hospital dosimeters standard.
Conclusion: To reduce the possibility of side effects of additional radiation, it is suggested that in the final absorption, which is determined for the patient during radiotherapy, doses be calculated for the area outside the treated area, too.
Keywords: mastectomy, dosimeters, tthermolumincence dosimetry (TLD), photovoltaic radiations, MV (Mega-Volt)
1The timing of the radiation sessions is determined by the treating physician
2 Photons resulting from the collision of electrons to slow down matter with the energy of 10 MeV
WHO, world health organization; TLD, thermoluminescence dosimetry; IAEA, international atomic energy agency; SSDLs, secondary standards dosimetry laboratories; SI, system of units; ECC, element correction coefficients
The most common cancer in women worldwide is breast cancer and radiotherapy is the best treatment choice after mastectomy.1 In the radiation therapy after mastectomy, depending on the type of tumor, the area of the breast removed undergoes radiotherapy. Since 1969, the International Atomic Energy Agency (IAEA), together with the World Health Organization (WHO), has performed postal TLD audits to verify the calibration of radiotherapy beams in countries.3 It is then important to reduce the side effects of the beam by determining the absorption dose in places outside the irradiation area. By determining the absorbing dose outside the irradiation region, future irradiation can be specified, so that absorption doses in areas outside the treatment area can be reduced to the lowest possible.
One of the important issues in medical radiotherapy today is to determine the dose absorption rate for the body of patients under radiation. In the design of the treatment, it is also important to determine the amount of absorbed dose in the areas that are outside the center of the x-ray or gamma radiation area. The data of this article was obtained from the Radiotherapy Department of Sayyed al-Shohada Hospital in Isfahan. This hospital follows an International Commission on Radiation Units and Measurements and standard protocols for the determination of the absorbed dose, these protocols are NCCN 2014, NICE and Evidence based oncology “Netherland”.4 Therefore, absorbed doses should be determined for the areas outside the center of the radiant curves. These curves are calculated and plotted by the standard institutions (Figure 1), for example, Secondary Standards Dosimetry Laboratories (SSDLs) of Iran provides calibrations for dosimetery equipment. SSDLs in Iran determine dose levels for patients, staff or public and also confirm the allowed absorbed dose level by the patient. Therefore, it is important to ensure that the measurement results are consistent with the International System of Units (SI).3
These curves could be used for measuring input doses, output doses, and intraperitoneal doses. Once in several years, to confirm these curves, SSDLs uses other techniques, such as dosimeters, MOSFETs, alanine dosimeters, film and dosimetry gels, and also in some cases, depending on the inherent characteristics of the type of detection, the type of measurement, and its availability, the type of detector is selected.5-15 The curves that are used in radiotherapy to calculate the patient's absorbed dose should be approved by SSDLs. In this article, these curves are called the Hospital Dosimetry Standard (HDS). Figure 1 shows a curve for calculating the absorbed dose of a region that was exposed to radiation gamma ray. As shown in this figure, in a 10×10 Cm2 range, for a distance of 3 Cm from the center of radiation, the absorbed dose will be 95%, for a distance of 6 Cm 10% and at the edge of the area, the absorbed dose will be 0%.5
In this study, TLD was used to measure the doses of absorption of radiation of the skin in the chest area in patients treated with the photovoltaic beams. TLDs have widespread uses in human dosimetery, radiotherapy and mammography. The use of the TLD was recommended with the support of the International Atomic Energy Agency (IAEA) and the World Health Organization (WHO) and the collaboration of hospitals in developing countries. Dosimetry methods have been improved in many hospitals in advanced countries with modern radiotherapy, such as Europe, the United States and Australia. It was suggested that the TLD be used to determine the absorbed dose more precisely.8 Materials of this kind are suitable for medical dosimetery for their properties, such as small size, fading rate of less than 5% per year. LiF with magnesium and titanium impurities is one of the materials used in making TLDs, which is (called the TLD100). This type of dosimeter has an atomic number of 7.4, which is close to the average atomic number of body tissue (8.2). Given the linearity of the absorbed dose curve, in the radiotherapy region, it is more appropriate for medical cases.5 The use of the TLD100 in determining the absorbed of dose has been common in Iranian hospitals for the past twenty years.6 The use of TLDs has also been presented in the form of research papers or projects. For example, the non-involvement of breast cancer in patients undergoing mastectomy breast cancer has already been examined in three hospitals in Tehran,16 the result of which showed that the most important factor in increasing the dose to the non-affected breast was the placement of the patient. In another study, the uniformity of dosage distribution was investigated in target volume in breast radiotherapy techniques after mastectomy.17 The findings suggested the use of photovoltaic radiations technique in the treatment of breast cancer. Another study compared the absorbed dose in lung tissue of breast cancer after mastectomy.18,19 Based on the determined absorbed dose of pulmonary and tumor tissue while also reducing radiation side effects in a patient undergoing radiotherapy, the absorbed dose was determined by TLD100 for 5 patients with breast cancer.20,21 Receiving the dose in non-affected areas depends on the size of the breast and the way the patient is exposed. In the area where the mastectomy patient was undergoing radiotherapy, we tried to focus on the exact determination of the dose for the smaller area (mastectomy and around it). We calculated the absorbed dose region of the mastectomy and around the mastectomy areas on a patient who has used TID100. Therefore, differences between the absorbed dose of the HDS and the TLD100 were observed.
These differences can be due to the scattering of the photovoltaic beams, the regulation of an irradiation device on the patient and the patient's physical condition. The advantage of studying and pursuing such research is that, in the end, each individual can be examined separately with regard to the necessary and unnecessary dosages, and a special radiotherapy program for each patient will be presented according to the state of the body and the irradiation facilities of the treatment center. Based on HDS, an 8×8 Cm2 area for a tumor at a depth of 3Cm has a 95% absorbed dose while 86% of doses is absorbed by the skin. According to the prescribed dosage of 200cGy per radiation therapy session, a simple fit curve can be used to calculate the dose received by the skin. For the radiation therapy program, the skin of the region should be treated with absorbent doses of 172cGy and for the areas outside the treatment areas, the skin should not receive any doses.
Experimental section: In this study, thermoluminescence dosimetery such as TLD100 chips were used5,7, to calculate the skin absorbed doses after mastectomy in breast cancer.10 The doses obtained by TLD100 were also compared with doses which were recorded in hospital (such as the curves in Figure 1). For reading TLD chips was used TLD Reader Harshow-4500, and the patient under radiotherapy in the project was a woman about 50years old with a tumor at a depth of 3Cm to the right of her chest, with the right breast being mastectomized. A patient is usually subjected to 25 irradiation sessions and at each session she receives 200 cGy doses in the treated area. In the course of the treatment, the patient received a dose of 5000 cGy per healing period according to the doctor's diagnosis. Figure 2 shows the areas that were irradiated by the photovoltaic 10MV under of mastectomy region. The mastectomy region of 20×20 Cm2 is irradiated, including the Intramammary regions (region 2 shown in Figure 2.) and Supraclavicular (region 1 in Figure 2). Supraclavicular and Intramammary regions were 20×8 Cm2 (region 1 in Figure 2), and 5×20 Cm2 (region 2 in Figure 2.) respectively.

Figure 1 A sample of the dose absorption curve from the perpendicular viewpoint of the cobalt radiation field.
This project, chips were read by the TLD reader at the University of Kashan with the Harshow-4500 model. For the dosimetry of the first irradiation areas, the element correction factor for each chip had to be obtained. Due to the limited number of chips, only twenty-seven TLD100s were used in this project. 27 TLD100s were subjected to gamma-ray radiation of 200cGy, and the Micro coulomb (µC) output and the Element Correction Coefficients (ECC) was measured. Each chip was determined according to equation (1) under the same conditions.
(1)
In Equation 1, the fractional form refers to the mean total of the count for each group ((Count)) and the denominator of this relation shows the count i (Counti) of the TLD100s. Because of the limited number of chips, they were categorized into 6 groups of 4, and a group of 3. Table 1 below shows the element correction coefficients obtained for each chip according to Equation 1.7,14
Chips number |
ECC |
Chips number |
ECC |
Chips number |
ECC |
Chips number |
ECC |
Chips number |
ECC |
1 |
1.167 |
7 |
0.68 |
13 |
1.007 |
19 |
0.945 |
25 |
0.962 |
2 |
1.55 |
8 |
1.026 |
14 |
1.106 |
20 |
0.887 |
26 |
0.782 |
3 |
1.364 |
9 |
0.919 |
15 |
0.791 |
21 |
1.186 |
27 |
1.176 |
4 |
1.088 |
10 |
0.743 |
16 |
0.749 |
22 |
1.106 |
|
|
5 |
1.107 |
11 |
0.966 |
17 |
1.084 |
23 |
1.101 |
|
|
6 |
0.74 |
12 |
0.783 |
18 |
1.027 |
24 |
1.089 |
|
|
Table 1 ECC for each TLD100 chips exposed to gamma rays
According to Table 1, the average percentage of changes in the chips was 72.3%. To determine the element correction factor for TLD100 chips in the range of photovoltaic radiation 10 MV and cobalt 60, the chips, without changing their numbers, were irradiated in the same way to 200cGy. The element correction coefficients obtained are shown in Table 2.
Chips number |
ECC |
Chips number |
ECC |
Chips number |
ECC |
1 |
1.167 |
10 |
0.743 |
19 |
0.945 |
2 |
1.55 |
11 |
0.966 |
20 |
0.887 |
3 |
1.364 |
12 |
0.783 |
21 |
1.186 |
4 |
1.088 |
13 |
1.007 |
22 |
1.106 |
5 |
1.107 |
14 |
1.106 |
23 |
1.101 |
6 |
0.74 |
15 |
0.791 |
24 |
1.089 |
7 |
0.68 |
16 |
0.749 |
25 |
0.962 |
8 |
1.026 |
17 |
1.084 |
26 |
0.782 |
9 |
0.919 |
18 |
1.027 |
27 |
1.176 |
Table 2 ECC after irradiation with the photovoltaic 10MV radiation
As shown in Tables 1 & 2, the ECC for both types of radiation for each chip were equivalent up to the third decimal point. Consequently, this ECC is the same for patients was under radiotherapy cobalt 60 radiation. In fact, this is an intrinsic property of TLD100 s. It can be concluded that the amount of gamma absorbed is the same as that of photovoltaic absorbance.
According to HDS, an 8×8 Cm2 field for a tumor at a depth of 3Cm has a 95% absorbed dose and a dosage that absorbs the skin is 86%. According to the prescribed dosage of 200cGy per radiation therapy session, a simple fit curve can be used to calculate the dose received by the skin. According to skin radiotherapy program, the treatment area should have an absorbed dosage of 172cGy, while the skin should not receive any doses. As shown in Table 3, none of the goals were met.
Location |
Dose measured using TLD (cGy) |
Calculated dose based on HDS (cGy) |
Difference |
1 |
5.39± 0.2 |
0 |
5.39 |
2 |
7±0.4 |
0 |
7 |
3 |
152.2±0.02 |
172 |
-19.48 |
4 |
148.08±0.09 |
172 |
-22.92 |
5 |
204.04±0.03 |
172 |
32.82 |
6 |
177.18±0.07 |
172 |
5.18 |
7 |
1.73±0.2 |
0 |
1.73 |
Table 3 The average dose calculated for each area
Results: In this project, first, the absorbed dose of radiation on the skin was obtained in terms of cGy of HDS (as shown in Figure 1), and then compared with the amount of absorbed dose as calculated by the TLD100s and their differences. The calculation process was as follows: the absorbed dose in the seven groups of chips was counted as cGy. Also, for the areas outside the field, the absorbed dose was measured (with three repetitions for each chip). Finally, with the average of each region, the numbers in Table 3 (average calculated doses) of each area were determined. Of course, as shown in Table 3, the calculated doses are matched with HDS. Based on the HDS of regions 1, 2, 7, the absorbance value is zero, once the TLD100s of the absorbed dose are numbers 5.39, 7, 1.73cGy, respectively. On the other hand, zones 3 and 4 had fewer doses, and in zones 5 and 6 HDS was higher. The difference is shown in Table 3. In addition, the numbered areas with their absorbed dose are shown in Figure 3.
As shown in Figure 4, the absorbed dose in the three regions 1, 2 and 7 should not absorb any amount of doses according to HDS, once the absorbed dose of 5.39, 7 and 1.73 cGy is observed.
In Figure 5 the white column in the corner of the chart (No. 0) is the absorbed dose recorded in HDS. With the help of this chart, we can see the difference in doses calculated with the HDS. Finally, the percentage of absorbed dose by TLD was compared with the percentage of dose absorbed by HDS. Table 3 shows the difference in absorbed dose in each group and the dose recorded in HDS is shown in Figure 6–8.

Figure 6 The absorbed dose diagram for each group in the first series of radiotherapy in terms of percentage difference.
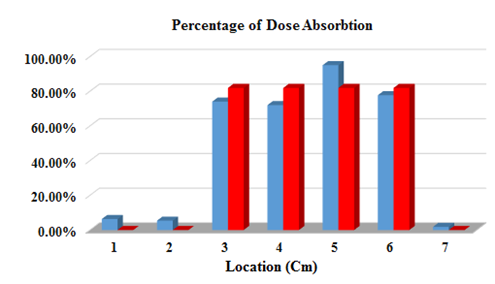
Figure 7 Shows the absorbed dose diagram of each group in the second series of radiotherapy in terms of percentage difference.

Figure 8 Shows the absorbed dose diagram for each group in the third series of radiation therapy in terms of percentage difference.
For regions 3, 4, 5, and 6, the average absorbed dose for each group for the first series is calculated and the dose recorded in HDS is shown in terms of percentage difference in Figure 6. The bars with Red color bars represent the absorbed dose recorded in HDS and the Blue bars indicate the dose absorbed by the TLD100s.
Figure 8 shows the average of the absorbed dose of each group for the third series of radiation therapy, and shows the dose in the dosimetry section in terms of the percentage difference of absorbed dose for each group of 3, 4, 5, and 6. Finally, the average absorbed dose of these three graphs is calculated as shown in Figure 9.
The graphs in Figures 6–9 show the difference in the computational theory of the HDS and TLD100 s results. Areas where the dosage of TLD100 s is greater than the dose for treatment can be determined by using the graphs in Figs. 6 to 9. Therefore, radiation modifications should be made to reduce the amount of unwanted absorbed dose of radiation. On the other hand, in most radiotherapy centers, the absorbed dose recorded in the HDS is based on values that are calculated from the solution of the theory of beam absorbed theory and its simulation in phantom. Therefore, with the numerical results obtained from the dosimetry measurements of the TLD100 s and their comparison with the results of the theory of HDS, for each of the irradiated points, the correction coefficients that match these two computational methods can be obtained.
The absorbed dose calculated with TLD100 at 1,2,5,6 and 7 points is higher than the dose absorbed in the skin, which is obtained using HDS. As noted above, points 1, 2 and 7 are located outside the area that is irradiated. However, in point 1,2,7, the skin absorbed 5.42, 5.19 and 1.85cGy more than the dose which was recorded, respectively. Of course, points 1 and 2 are almost near radiation areas, where there is the possibility of unpredictable absorption because of the error in placing the patient or radiation. But point 7, which is far apart from both areas of radiation, is still observed for non-zero absorbed doses, which can be due to the photovoltaic scattering at irradiation. At points 3 and 4, the dose absorbed by the skin is lower than the absorbed dose recorded in the HDS. The reason for this can be because of the use of applicators,21 which absorbs more doses at one point and fewer doses at other points. Regardless of the areas in which the dosimetry of the Drug Treatment Center is zero, the computational dose differed by HDS computing amounts of a thermoluminescence series of an average of about 7.57%. Therefore, it is recommended that more extensive research be done on the implantation of shields in uncapped areas in the form of treatments to reduce the patient's ineffective absorbed dose. On the other hand, mastectomy requires 25 sessions of radiation, and we observed the variation in dose results absorbed by TLD100 s with HDS. So with the dosimetry of TLDs, the side effects that are caused by the unnecessary radiation absorbed could be minimized. Similarly, by comparing the results obtained from numerical and metering software for absorbed doses, it is possible to reduce the difference between theoretical and practical dose reduction, and ultimately improving clinical radiotherapy. In the end, it is recommended this project be implemented, for large number of people that have had underwent radiotherapy treatment. Therefore, in order to be more precise based on the statistical data we can design a more accurate absorbed dose for the involved and non- involved areas.
We’d like to thank Dr. Mahnaz Roayaei from Radiotherapy Center of Isfahan Seyed al-Shohada Hospital. She received the medical consultation on this project. We are also obliged to thank the Nuclear Department at Kashan University, which provided the TLD reader.
Author declares there are no conflicts of interest towards the article.

©2023 Ali, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.