eISSN: 2373-6372


Review Article Volume 5 Issue 3
1Department of Gastroenterology, Hospital 0 Aout, Morocco
2Department of Epidemiology and Biostatistics, School of Medicine and Pharmacy of Tangiers, Morocco
3Department of Medical Oncology, University Hospital Hassan II, Morocco
Correspondence: Nada Lahmidani, Department of Gastroenterology, Hospital 20 Aout, 9 bloc A Hay Hassani Erac, Fès, Morocco
Received: August 27, 2016 | Published: September 22, 2016
Citation: Lahmidani N, Najdi A, Benbrahim Z (2016) Update on Clinical and Epidemiological Features of Viral Hepatitis A. Gastroenterol Hepatol Open Access 5(3): 00144. DOI: 10.15406/ghoa.2016.05.00144
Hepatitis A is considered as a contagious liver disease due to the infection by Hepatitis A virus (HAV). It is the most common form of acute viral hepatitis in the world. Geographic disparities in the distribution of the infection are essentially related to hygienic and socioeconomic conditions. Let’s see an overview of the biological, epidemiological and clinical characteristics of this infection.
Identification of the causative agent
Hepatitis Virus A is a hepatovirus belonging to the family of Picornaviridae, characterized by single-standard, positive sense, linear RNA measuring 27nm and non-enveloped.1,2 The virus was first isolated by Purcell in 1973in the stool of patients by electron microscopy allowing future serologic testing discovery. HAV displays a high degree of antigenic and genetic conservation.1,3,4 Only a single serotype is described. In 1995, an effective vaccine to prevent HAV infection was available in the United States and in 2005, the disease becomes notifiable.2,5
Viability and resistance
HAV is a very resistant virus in the environment because of by the absence of envelop. The virus is resistant against denaturation by drying, temperature as high as 56°C and as low as -20°C and acid at pH 3.0.3,5,6 The virus can also survive in used water for weeks at 20°C temperature especially if the humidity is low. The virus is neutralized after heating at 70°C for 4 min and inactivated by radiation, by the glutaraldehyde 2%, formaldehyde and bleach reconstituted and diluted at 1/5.5,7
Genotyping
Three genotypes of the HVA have been described in Humans. These genotypes are divided into subtypes A and B.7 Genotype I is the most frequently reported worldwide, particularly genotype IA (North, Central and South America, China, Japan, Thailand and Europe). Subtype IB is mainly observed in the Mediterranean region, South Africa and Turkey. In India, Nepal and Korea, the sub genotype IIIA is the most frequent one. We should particularly note the viral diversity in Europe. Genotypes IIA, IIB and IIIB are not frequently reported.8,9
Reservoir and vector
Man and certain primates are the only HAV reservoir but man is the main reservoir. Monkeys can be infected, but it seems that man is unreceptive to simian strains.2 Transmission is primarily by direct contact with infected person with fecal-oral route (person to person). It can also be indirect via contaminated water or foods (shellfish) especially in countries with low levels of hygiene. Transmission through infected blood transfusion or among injecting drug users has been described in very rare cases. Intersexual transmission has been reported in gay men (oral-anal practices). Vertical transmission is described but is rare.5–7
Unlike hepatitis B and C, there is no chronic form of hepatitis A. The disease is expressed in an acute symptomatic form, asymptomatic or complicated form. Symptoms are benign in 95% cases, but rarely,2 (1 case / 10000) the disease can be expressed in a fulminant and fatal form (adults and elderly).1,2,10
The symptomatic acute form Mild1,5,6,10
The evolution of the disease is organized in 4 periods. The Incubation period lasts 30 days on average depending on infective load. The pre jaundice phase that lasts 1 to 3 weeks and characterized by nonspecific symptoms like fever, loss of appetite, nausea and vomiting, abdominal pain, asthenia, arthralgia and myalgia, weight loss and more rarely hives. The jaundice phase is characterized by the appearance of mucocutaneous jaundice present in 70-85% of adults. It lasts a few days to several weeks. Jaundice is accompanied by clay-colored stool, dark urine and rarely pruritus. Sometimes hepatomegaly is present (40%). Jaundice resolves within few weeks. Finally, the disease progresses to a convalescence period with a favorable clinical outcome.
Other clinical forms11–13
Asymptomatic and extra hepatic forms: Symptomatic forms with extra hepatic manifestations are frequent with or without elevated transaminases. Asymptomatic forms are more common in children (over 90% of children under the age of 5 years).
Prolonged cholestatic forms: Are seen in 15% of cases and can last several weeks to several months. The patient has asthenia +/- jaundice. No chronicity has been described.
Relapsing forms: Their prevalence is highly variable (1-20% of patients with acute hepatitis) and may occur 3-9 months after clinical recovery.
Fulminant form: 1 case / 10.000: This form is more common in adults, elderly person aged more than 60 years (1.5%) and in patients with underlying chronic liver disease. It is characterized by impaired consciousness; severe hemorrhagic syndrome with severe liver failure that may require liver transplantation. Spontaneous fatality rate is 70-90%.
Complicated forms: Rare: The evolution can be characterized by the occurrence of several complications: Acute renal failure, interstitial nephritis, pancreatitis, red blood cell aplasia, Guillain Barré syndrome, still disease, etc.
Nonspecific laboratory tests show an increase of transaminases serum level, sometimes very marked (20 to 40 times the normal level), predominantly on ALAT. A hyperbilirubinemia is often present. Prothrombin time may be normal or lowered in relation to the liver failure.2 Culture requires specific environment and is non-routinely performed. Search for viral RNA in blood or stool is limited to clinical studies. The diagnosis of acute Hepatitis A is based on the detection of immunoglobulin M appearing early (5 to 10 days after exposure), and reaching a maximum rate 60-90 days after contagion and may persist for up to 6 months. The presence of IgG indicates a past infection or acquired immunity.2,4
Infectivity
The infectious associated risk with hepatitis A virus belongs to the class 2. The disease is notifiable since 2005.It is a strict pathogenic human virus. The infectious dose is low (10-100 viral particles).2,3 The more infectious dose, the earlier is the onset of the clinical signs (dose-response effect). The virus is one of the most common causes of food borne infections. Outbreaks of infection may declare secondary to contamination of water and food and persist for a long time. The pic of infectivity is 2 weeks before onset of jaundice.14,15 A person infected with HAV is contagious latter half of the incubation period and for several days after onset of jaundice. Prolonged excretion of the virus is possible in children and infants. Cases are considered noninfectious 7 days after onset of jaundice.3,16–18
Case fatality-lethality
Death from hepatitis A is rare. Nevertheless, it is more frequent in patients more than 60 years and those with underlying liver disease. One hundred people die every year of acute liver failure post HAV infection in US.15–18 The case fatality is about 0.3% and increase to 1.8% in risky person. Of the 1,562 case reports of hepatitis during 2012, 0.6% died from complications of the disease. In 2011, Asian/and Pacific Islanders had the highest mortality rates (0.06 deaths per 100,000 population) with same rates for males and females.5,6,15
Pathogenicity
The acquisition of HAV occurs often through fecal-oral transmission .The virus is then transported across the intestinal epithelium to join the hepatocytes via the mesenteric veins. The virus starts replicating within the cytoplasm via RNA dependent polymerase. The liver injury (diffuse liver necrosis and cholestasis) is probably a consequence of cell mediated immune response (Natural killers, CD8+, Tlymphocytes). Severe hepatitis is explained by a dramatically host response.HAV is shed after to the bile canaliculi and then to intestines through bile and then fecal excretion.16,19
Virulence
HAV is considered as the least serious viral hepatitis. The body combats it in a few weeks and immunity is for life. Some studies have suggested potential emergence of antigenic variants in HAV that might influence replication of the virus, and thereby affect virulence.14,20 The severity of the disease depends on the age of infection and the preexistence of liver disease.13
Case definition
A confirmed case is a case that meets clinical and biological definition or meets clinical definition and occurs in a person with epidemiological link with confirmed case.15,16,20 Clinical definition: An acute illness with discrete onset of any sign of acute viral hepatitis (fever, asthenia etc…) and either jaundice or increased transaminase level. Biological definition: positivity of Immunoglobulin M antibody to HAV.
Immunogenicity
Every person recovering from acute HAV develops IgG antibodies which appear early during the infection and provide lifelong protection against the disease.4,5
Descriptive statistics
Incidence rate-Seroprevalence: HAV is distributed worldwide as sporadic or endemic cases; it represents 20-25% of cases of hepatitis in the world. The estimated number of new cases of hepatitis A in the world is about 1.4 million new cases per year.17,19,20 Distribution of hepatitis A cases reflects the disparities in the level of hygiene and socio economic development in the world. The disease spread in endemic form in developing countries. HAV prevalence is estimated at 15% at the age of 30 years in the world.17 In the US, the incidence of HAV varies in a cyclic pattern. Outbreak disease occurs every 5-10 years. US are an area of low endemicity.15,21,22 One third of the US population has been infected in the past. The overall incidence rate for 2012 was 0.5 cases per 100,000 populations (1,562 acute hepatitis A reported cases). Disparities exist between states. The case rate ranged from 0 cases in South Dakota to 1.4 cases per 100,000 populations in Arizona.22,23
Geographic distribution: We distinguish 3 distinct area of the world regarding prevalence of hepatitis A.23,24–28
Sex-age distribution: No sexual predilection is apparent except for certain person at high risk like male homosexuals. The age of acquisition of the infection differs according to geographic areas as we have seen before (in high seroprevalence area, 90% of children less than years are seropositive).20,26,27 As the age of acquisition increases, the disease is more frequent, more symptomatic and complications and mortality are increased. In US, in 2012, rates were highest for persons aged 20-29 years (0.69 cases per 100,000 populations) .The incidence rate was 0.5 cases per 100,000 population the same for males and females.15,25 Studies of migrants have shown that immigrants from area with high endemicity to area with low rates explain in part the cyclic pattern of VHA outbreaks.26
Mortality rate: In US in 2011, the CDC reported a mortality rate of 0.02 deaths per 100,000 populations higher among persons aged ≥45 years.21
Time trends: Incidence of VHA is worldwide decreasing since last 20-30 years particularly in developed countries because of the improvement of sanitary, hygienic conditions, socioeconomical status, lifelong immunity and the introduction of vaccination in some countries like Italy, Spain (Catalonia) and US.17,19 The CDC, reported that the incidence of Hepatitis A declined by 90% between 1995 and 2006 to the lowest rate ever recorded especially in states where the vaccination of children was recommended (Figure 1).6,15,25
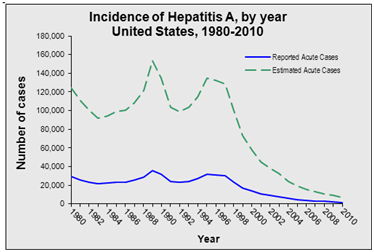
Figure 1 The Decline in Hepatitis A infection since the introduction of the vaccine in 1995 in US.5
Population at risk
People at risk are those traveling or working in countries with high or intermediate rates of HVA, living in a community with poor hygienic conditions, men having sex with men, users of injection drugs, those with occupational risk: pediatric services, child care facilities, institutions for the disabled, laboratory technician and staff performing work in contact with water use and finally Household members.17–19,25,28
None.
The authors declare no conflicts of interest.
None.

©2016 Lahmidani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.