eISSN: 2373-6372


Review Article Volume 15 Issue 5
Pharmacology Department, Medical Research and clinical studies institute, National Research Centre, Egypt
Correspondence: Manal M E Ahmed, Pharmacology Department, Medical Research and clinical studies institute, National Research Centre, Giza, Egypt, Tel +20 1093627027
Received: November 14, 2024 | Published: November 22, 2024
Citation: Ahmed MME. STAb CAR-T Cells: pioneering precision in cancer immunotherapy. Gastroenterol Hepatol Open Access. 2024;15(5):141-145. DOI: 10.15406/ghoa.2024.15.00591
Chimeric Antigen Receptor (CAR) T cell therapy has revolutionized cancer treatment, particularly for hematologic malignancies. However, challenges such as limited efficacy in solid tumors and antigen escape have prompted the development of novel approaches. One such innovation is the STAb (Synthetic T-cell Activating Bifunctional) CAR-T cells, which aim to enhance the therapeutic potential of CAR-T cells. This review will investigate the functioning, benefits, clinical trials, obstacles, and future prospects of STAb CAR-T cells, underscoring their transformative potential in cancer treatment.
Chimeric Antigen Receptor (CAR) T cell therapy has emerged as a groundbreaking treatment for certain types of cancer, particularly hematologic malignancies such as leukemia and lymphoma. This innovative approach involves genetically modifying a patient’s T cells to express CARs, which are engineered receptors designed to target specific cancer antigens. Upon reinfusion into the patient, these CAR-T cells can recognize and destroy cancer cells with remarkable precision.1,2 Despite the success of CAR-T cell therapy in blood cancers, its application in solid tumors has been met with significant challenges. Solid tumors present a more complex microenvironment, characterized by physical barriers, immunosuppressive factors, and heterogeneous antigen expression. These factors contribute to the limited efficacy of traditional CAR-T cells in treating solid tumors.3,4
To address these limitations, researchers have developed Secreted Tumor Antigen-Binding (STAb) CAR-T cells. This novel approach aims to enhance the therapeutic potential of CAR-T cells by incorporating synthetic antibodies that can bind to multiple tumor antigens simultaneously. By targeting a broader range of antigens, STAb CAR-T cells increase the likelihood of recognizing and attacking cancer cells, even if they downregulate one or more antigens to evade immune detection.5
The development of STAb CAR T cells represents a significant advancement in the field of cancer immunotherapy. By overcoming some of the key obstacles faced by traditional CAR-T cells, STAb CAR-T cells hold promise for more effective and versatile cancer treatments. This review will explore the mechanism of action, advantages, clinical trials, challenges, and future directions of StAb CAR-T cells, highlighting their potential to revolutionize cancer therapy.6,7
Mechanism of action
CAR-T (Chimeric Antigen Receptor T-cell) therapy is a form of immunotherapy that utilizes a patient’s own immune system to combat cancer. The process starts with collecting T cells from the patient’s blood. These T cells are then genetically engineered in the lab to express chimeric antigen receptors (CARs) on their surface, which are designed to recognize and bind to specific antigens on cancer cells. After modification, the T cells are expanded in the lab to produce a sufficient quantity of CAR-T cells. These cells are then infused back into the patient, where they seek out and attach to cancer cells. Upon binding, the CAR-T cells become activated and release cytotoxic molecules like perforin and granzymes, which induce apoptosis (programmed cell death) in the cancer cells. The CAR-T cells continue to proliferate and remain in the body, providing ongoing surveillance and destruction of cancer cells, thereby helping to prevent relapse and maintain remission. However, CAR-T cell therapy has several limitations. One significant challenge is the potential for severe toxicities, such as cytokine release syndrome (CRS) and neurotoxicity, which can occur when the immune system is overly activated. Additionally, CAR-T cell therapy has shown limited efficacy against solid tumors compared to blood cancers. This is due to factors like the lack of tumor-specific antigens, the heterogeneous nature of solid tumors, and the immunosuppressive tumor microenvironment that can inhibit CAR-T cell function. Cancer cells can also evade CAR-T cell therapy by downregulating or mutating the target antigen, a phenomenon known as antigen escape. Furthermore, CAR-T cells often face difficulties in trafficking to and infiltrating solid tumor sites due to the physical barriers of the tumor microenvironment. The manufacturing process of CAR-T cells is complex and time-consuming, involving multiple steps that can be lengthy and costly. Lastly, the immunosuppressive tumor microenvironment in solid tumors can inhibit the activity of CAR-T cells, making it challenging for them to maintain their efficacy within the tumor.
STAb CAR-T cells offer several advantages over traditional CAR-T cells. One key benefit is their ability to recruit and activate other T cells in the body that have not been genetically modified, thereby amplifying the overall immune response against the cancer. This amplification enhances the effectiveness of the therapy. Additionally, STAb CAR-T cells are designed to overcome the immunosuppressive tumor microenvironment, ensuring a more effective and sustained attack on cancer cells. These cells also address the issue of antigen escape by targeting multiple antigens, reducing the likelihood of cancer cells evading the therapy. Mechanistically, while traditional CAR-T cells rely mainly on the CAR interaction with the tumor antigen, StAb CAR-T cells more closely mimic the natural T-cell receptor (TCR)-mediated immune synapse, which enhances their stability and strength. Overall, the combination of targeted killing, immune system amplification, and the ability to overcome the tumor microenvironment makes STAb CAR-T cells a promising approach in the fight against cáncer (Figure 1).8
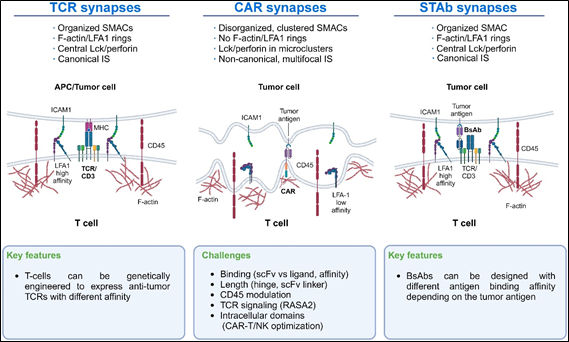
Figure 1 Immunological synapse features of TCRs, CAR-T cells, and STAb-T cells: The main differences in the IS of TCRs, CAR-T cells, and STAb-T cells are shown. These differences could guide new lines of research to improve CAR-T cells’ IS formation.8
Comparative analysis between StAb CAR-T cells and traditional CAR-T cells
In brief, While traditional CAR-T cells have revolutionized the treatment of certain blood cancers, they face challenges such as limited persistence and potential relapse. StAb CAR-T cells, with their ability to secrete bispecific antibodies and target multiple antigens, offer a promising alternative with enhanced persistence and broader applicability, particularly in cancers like multiple myeloma. This innovative approach not only improves the direct targeting of cancer cells but also recruits the body’s natural immune cells to join the fight, potentially leading to more effective and lasting cancer treatments.
StAb CAR-T advantages over traditional CAR-T cells
In brief, STAb CAR-T cells are designed to form a more stable and stronger immune synapse by mimicking key features of the TCR-mediated synapse more closely. This improved synapse formation enhances the activation and persistence of the T cells, leading to a more robust and sustained immune response against the tumor. These features make STAb CAR-T cells a promising advancement in the field of cancer immunotherapy, potentially offering more effective and durable treatments for patients.8
Clinical Trials and Research: STAb CAR-T cells represent a significant advancement in cancer immunotherapy, particularly for hematological malignancies like multiple myeloma. These cells are engineered to target specific antigens on cancer cells, similar to traditional CAR-T cells, but with some notable differences. Recent clinical trials have demonstrated the potential of STAb CAR-T cells in various cancer types. For instance, a study published in Science Translational Medicine highlighted their effectiveness in multiple myeloma, outperforming traditional CAR-T cells by amplifying the immune response.10 One of the key findings from recent studies is the enhanced efficacy of STAb CAR-T cells. They have demonstrated higher effectiveness compared to traditional CAR-T cells in preclinical models. This increased efficacy is partly due to their ability to recruit natural, unmodified T cells within the body to attack tumor cells, thereby amplifying the therapeutic effect. Another important aspect of StAb CAR-T cells is their improved persistence. One of the challenges with CAR-T cell therapy is ensuring that the cells remain in the body long enough to provide a lasting therapeutic effect. STAb CAR-T cells have shown better persistence, which is crucial for achieving long-term remission in patients. Additionally, these cells can be engineered to target multiple antigens, potentially reducing the likelihood of cancer cells escaping detection and leading to more comprehensive treatment outcomes.11
STAb CAR-T cells have been the focus of several preclinical and clinical trials, showcasing their potential in treating various cancers. In preclinical studies, researchers have explored the efficacy of STAb CAR-T cells in targeting hematological malignancies. One notable study involved T cells engineered to express an anti-CD22 CAR and secrete an anti-CD19 T-cell engager antibody. This dual-target strategy was tested against B-cell acute lymphoblastic leukemia (B-ALL) and demonstrated superior therapeutic potential compared to traditional CAR-T therapies. The preclinical trials included both in vitro and in vivo models, where the STAb CAR-T cells showed enhanced tumor cell killing and improved persistence. Another preclinical study focused on cortical T cell acute lymphoblastic leukemia, where StAb-T cells targeting CD1a were highly effective in both laboratory and animal models.
The NEXT CAR-T Consortium in Madrid is at the forefront of clinical trials involving STAb CAR-T cells. This consortium aims to develop novel cell-based immunotherapies for relapsed or refractory cancers. Their multicenter clinical trials are evaluating the safety and efficacy of these therapies in patients with poor prognosis cancers. One of their significant efforts includes trials for multiple myeloma, where STAb CAR-T cells have shown promising results by recruiting additional immune cells to enhance the anti-tumor response. Additionally, larger pharmaceutical companies are also involved in developing in vivo CAR-T programs, which include STAb CAR-T cells. These programs aim to reprogram immune cells directly within the body, potentially making the therapy faster, more effective, and less expensive.8
These trials highlight the potential of STAb CAR-T cells to revolutionize cancer treatment by offering more effective and durable responses, especially for patients with difficult-to-treat malignancies. The ongoing research and clinical evaluations continue to provide hope for advancements in cancer immunotherapy.
Challenges and future directions
Challenges
CAR-T cells might target healthy tissues that express low levels of the same antigens as cancer cells, leading to "on-target, off-tumor" effects. This can cause damage to vital organs and tissues, resulting in severe side effects. To improve targeting specificity, researchers are developing dual-specific CAR-T cells that require the simultaneous recognition of two different antigens, reducing the likelihood of attacking healthy cells. Another approach is the use of "safety switches" or "suicide genes" within CAR-T cells that can be activated to eliminate the cells if severe side effects occur. Additionally, researchers are investigating the use of advanced screening techniques to identify antigens that are exclusively or predominantly expressed on cancer cells.15
Future directions
By addressing these challenges, STAb CAR-T cell therapy can become a more effective and widely applicable treatment for various types of cancer. The combination of targeted killing, immune system amplification, and the ability to overcome the tumor microenvironment makes STAb CAR-T cells a promising approach in the fight against cancer.
Concluding remarks
In summary, STAb CAR-T cells represent a transformative advance in cancer immunotherapy, offering a novel approach to targeting and eradicating cancer cells with unprecedented precision. By harnessing the dual capabilities of T-cell activation and antigen targeting, these bifunctional cells address key limitations of traditional CAR-T cell therapies, including insufficient tumor infiltration, antigen escape, and off-target effects. The integration of ongoing monitoring and adaptive modifications further enhances the efficacy and safety profile of STAb CAR-T cells, making them a powerful tool in the fight against both hematologic malignancies and solid tumors.
Despite the promising potential, several challenges remain, including the complexity of production, managing adverse events such as cytokine release syndrome, and ensuring long-term efficacy. Continuous research and clinical trials are essential to refine these therapies, optimize their delivery, and expand their applicability. As innovations in genetic engineering, combination therapies, and personalized treatment strategies advance, STAb CAR-T cells are poised to become a cornerstone of precision oncology, offering new hope for patients and paving the way for a future where cancer can be more effectively controlled and potentially cured.

©2024 Ahmed. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.