eISSN: 2373-6372


Research Article Volume 9 Issue 5
1Tropical Medicine Department, Ain Shams University, Egypt
2Internal Medicine Department, Ain Shams University, Egypt
Correspondence: Mohamed Omar Khalifa, MD, Assistant Professor of Tropical Medicine, Ain Shams University, Abbasia, Cairo, Egypt, Tel +20 2 22871187, Fax +20 2 26820665
Received: July 19, 2018 | Published: September 18, 2018
Citation: Khalifa MO, Ahmed OA, Safwat E. Prognostic role of serum alpha-fetoprotein in hepatocellular carcinoma patients with radiofrequency ablation. Gastroenterol Hepatol Open Access. 2018;9(5):161-166. DOI: 10.15406/ghoa.2018.09.00317
Background/purpose: Prognostic value of serum alpha-fetoprotein (AFP) in hepatocellular carcinoma (HCC) is still debatable. We aimed to study this role in HCC patients who underwent radiofrequency ablation (RFA).
Methods: Records from HCC patients were retrospectively analyzed between January 2011 and December 2015. A minimum data set for each patient record of a follow-up period of at least 1 year was pre-defined before enrollment. In all, 153 patients were enrolled. AFP levels were recorded for all patients at the time of diagnosis, 1 month after RFA and at 3-month intervals afterward. Patients were divided according to pretreatment AFP level into 3 groups: group 1: AFP <20ng/mL, group 2: AFP 20-200ng/mL and group 3: AFP >200ng/mL.
Results: Pretreatment AFP is not significantly correlated with age, baseline lesion number or size, baseline Child score or class, post RFA recurrence or death. The overall survival rates were 95%, 75.6%, 55.6%, 48.8%, and 48.8% at 1, 2, 3, 4, and 5years respectively. On comparing the 3 groups on disease-free survival, there was no statistically significant difference among the three classes. Child class A patients showed statistically significant better survival after RFA than those with Child class B. The ROC curve showed that AFP had inadequate accuracy to discriminate survivors and deceased patients and to discriminate patients with recurrence from those without recurrence.
Conclusion: AFP level could not be used as a good predictor of either death or recurrence of HCC after RFA.
Keywords: alpha-fetoprotein, hepatocellular carcinoma, prognosis, survival
AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; AASLD, American association for the study of liver diseases; DFS, disease-free survival; OS, overall survival
Alpha-fetoprotein (AFP) is considered the most thoroughly investigated marker for diagnosing hepatocellular carcinoma (HCC). However, it has a limited diagnostic performance for the surveillance of HCC. 2 reasons may explain this; first, high AFP levels could be seen in patients with chronic hepatitis and liver cirrhosis,1 second, only a small proportion of early-stage HCCs (10–20%) present with increased AFP levels.2
The American Association for the Study of Liver Diseases (AASLD) guidelines for HCC diagnosis and treatment, however, has recently eliminated AFP measurement from the surveillance armamentarium because of its poor sensitivity and specificity for the diagnosis of HCC.3 Also, AFP assessment is not included in The Barcelona Clinic Liver Cancer (BCLC) classification system, although it has been identified by several studies as an overall independent predictor of survival.4
However, most of studies about the prognostic value of AFP have included heterogeneous cohorts of patients, thus preventing a proper evaluation of its performance as a prognostic marker in a selected subset of patients.5
In this study, we aimed at evaluating the prognostic role of AFP in patients with HCC treated with radiofrequency ablation (RFA).
This retrospective study was conducted at the HCC and Herpetology clinics of the Tropical and Internal Medicine Departments, Aim Shams University Hospitals, Cairo, Egypt. All patients with HCC who were diagnosed and underwent radiofrequency ablation in the period between January 2012 and December 2016 were reviewed and the data from the patients who fulfilled the inclusion criteria were retrospectively retrieved from their files.
The minimum data set within the patient record with a 1-year follow-up period was predefined before collection of data to be included as a record in this retrospective study. This study was confirmed to meet the standards of the Declaration of Helsinki and current ethical guidelines and was approved by the Research and Ethics Committee of Ain Shams University, Cairo, Egypt, in accordance with local research governance requirements.
Incomplete files or patients who did not complete a follow-up period of 1year were excluded from the study.
The main characteristics of the database have been previously reported. Our database includes patient demographics, main biochemical and hematological parameters, etiology and stage of liver disease, the presence of co morbidities, baseline and serial measurements of AFP, HCC stage and treatment, patient survival, and mortality.
Among all treated HCC patients in our department from January 2012 to December 2016, patients who fulfill the following criteria were only included:
Patients with advanced liver disease (Child-Pugh class C) or those with extra-hepatic metastasis or gross vascular invasion and those patients with any previous HCC treatment were excluded from this study. We calculated Disease-free survival (DFS) from the time of complete response to a curative procedure to the time of disease recurrence. Overall survival (OS) was calculated from the time of intervention to the date of death or that of the last follow-up visit (December 2017).
Analysis of survival was done at 1, 3, and 5years after treatment. The maximum tumor diameter was the proposed tumor size. In case of multiple tumors, the size was measured as the sum of the maximum diameters of all tumors. Follow up of all patients, with the measurement of serum AFP and CTs, was done every 3months in the first year after treatment, and then every 6 months for the next 4years.
Patients' informed consent to the study was not a requirement because their records were reviewed retrospectively and the clinical data that were obtained after each patient agreed to RFA by informed written consent before intervention.
Statistical analysis
Statistical analysis was performed with SPSS software (SPSS Inc., Chicago, IL, USA). Data were expressed as the mean±SD, median or count and percentage. Differences in continuous variables between the different groups' data were assessed by independent t-test. Mann-Whitney U tests, Kruskal–Wallis tests or χ2 tests were used to compare non-parametric variables. A level of significance (p) less than 0.05 was significant. One-way analysis of variance (ANOVA) was applied to compare all groups on quantitative variables to determine significant differences. Pearson correlations were used to assess the correlation between parameters of interest. Pearson correlation coefficients point to a direct correlation, while negative values point to an inverse correlation and were considered significant at the 0.05 level. Univariate regression analysis was used to assess the correlations of the predictors of death or recurrence. Life tables and Kaplan-Meier curves were used to present survival. The log-rank test was used to compare survival times between the different groups.
The probability of the AFP level predicting death or recurrence was used to construct receiver operating characteristic (ROC) curve. The efficacy of each panel was assessed by using area under the curve (AUC). As the AUC of AFP predicting death or recurrence did not reach a statistically significant level, no optimal cut-off values were selected.
According to pretreatment AFP level, patients were divided into 3 groups; group 1 included 59 patients (49 males and 10 females) with AFP less than 20ng/ml, group 2 included 54 patients (43 males and 11 females) with AFP levels of 20-200ng/ml and group 3 included 40 patients (30 males and 10 females) with AFP above 200ng/ml.
Table 1 shows the main demographic, clinical and tumor characteristics of the 153 study patients. The patients' mean age was 56.43±7.02years, and approximately 80 % of them were males. Hepatitis C virus was the main underlying etiology of liver cirrhosis (n=142, 92.8%). Around two-thirds of the patients were of Child-Pugh class A, and three-quarters of the patients had a single lesion.
Variable |
N (%) |
|---|---|
Age (years) |
|
Mean±SD |
56.43±7.02 |
Range |
42-76 |
Sex |
|
Male |
122 (79.7) |
Female |
31 (20.3) |
Viral infection |
|
HCV positive |
142 (92.8) |
HBV positive |
5 (3.3) |
HBV/HCV co-infection |
4 (2.6) |
Both negative |
2 (1.3) |
Child-Pugh class |
|
Class A |
104 (67.9) |
Class B |
49 (32.1) |
Child-Pugh score |
|
5 |
55 (35.9) |
6 |
49 (32.1) |
7 |
28 (18.3) |
8 |
19 (12.4) |
9 |
2 (1.3) |
Level of AFP (ng/ml) |
|
<20 |
59 (38.6%) |
20-200 |
54 (35.3%) |
>200 |
40 (26.1%) |
Tumor Characteristics |
|
Number of lesions |
|
Single lesion |
115 (75.2) |
Multiple lesions |
38 (24.8) |
Diameter of the largest lesion (cm) |
|
< 3 cm |
29 (19) |
≥3 cm |
124 (81) |
Table 1 Baseline Characteristics of Patients (N= 153)
The maximum diameter of the HCC lesion was ≥3cm in 124 patients (81%). Serum AFP levels were within the normal range (<20ng/ml) in 59 patients (38.6%), mildly elevated (20-200ng/ml) in 54patients (35.3%), and markedly elevated (>200ng/ml) in 40patients (26.1%).
In the present study, tumor recurrence was recorded in 88 cases (57.5%), and 53 (34.6%) patients died during follow-up. Comparison between the 3 groups on gender, age, lesion number, size of the largest lesion, Child class and score, recurrence and death revealed no significant differences between the three groups for any of the parameters as shown in Table 2.
|
|
AFP level (ng/ml) |
|
|
|
|
χ2 |
Sig |
|
|---|---|---|---|---|---|---|---|---|---|
<20 |
|
20-200 |
|
>200 |
|||||
|
|
Count |
% |
Count |
% |
Count |
% |
|
|
Gender |
Male |
49 |
40.20% |
43 |
35.20% |
30 |
24.60% |
0.957 |
0.62 |
Female |
10 |
32.30% |
11 |
35.50% |
10 |
32.30% |
|||
Child class |
A |
38 |
36.50% |
39 |
37.50% |
27 |
26.00% |
0.791 |
0.673 |
B |
21 |
42.90% |
15 |
30.60% |
13 |
26.50% |
|||
Child score |
5 |
22 |
40.00% |
18 |
32.70% |
15 |
27.30% |
0.063 |
0.969 |
6 |
16 |
32.70% |
21 |
42.90% |
12 |
24.50% |
|||
7 |
12 |
42.90% |
8 |
28.60% |
8 |
28.60% |
|||
8 |
8 |
42.10% |
7 |
36.80% |
4 |
21.10% |
|||
9 |
1 |
50.00% |
0 |
0.00% |
1 |
50.00% |
|||
Lesion number |
Single tumor |
49 |
42.60% |
37 |
32.20% |
29 |
25.20% |
3.395 |
0.183 |
Multinodular |
10 |
26.30% |
17 |
44.70% |
11 |
28.90% |
|||
Recurrence |
Disease-free |
23 |
35.40% |
21 |
32.30% |
21 |
32.30% |
2.224 |
0.329 |
Recurrence |
36 |
40.90% |
33 |
37.50% |
19 |
21.60% |
|||
Death |
Alive |
39 |
39.00% |
33 |
33.00% |
28 |
28.00% |
0.825 |
0.662 |
Dead |
20 |
37.70% |
21 |
39.60% |
12 |
22.60% |
|||
Mean |
±SD |
Mean |
±SD |
Mean |
±SD |
F |
Sig |
||
Age (years) |
57.153 |
6.7487 |
56.444 |
7.6938 |
55.35 |
6.4987 |
0.782 |
0.459 |
|
Size of largest lesion (cm) |
3.29 |
0.9484 |
3.426 |
0.8785 |
3.407 |
0.8669 |
0.37 |
0.691 |
|
Table 2 Comparison between the 3 groups regarding different parameters
The correlation between AFP and other variables (age, size, number of lesions, Child score, and class, recurrence, and death) revealed that pretreatment AFP was not significantly correlated with age, baseline lesion number or size, baseline Child score or class, post RFA recurrence or death.
The overall survival intervals (time to death or end of the study in months), were 95%, 75.6%, 55.6%, 48.8%, and 48.8% at 1, 2, 3, 4, and 5years, respectively. The mean survival interval was 33.6months in group 1, 34.3months in group 2, and 28.6months in group 3 with no evidence of significant differences between the three groups (p=0.207).
Figure 1 shows the Kaplan-Meier survival curves of all groups. No significant differences was noticed among the three AFP classes in overall survival (χ2=1.846, P=0.397). The median recurrence-free interval was 28, 28 and 35 months in group 1, 2 and 3; respectively (p=0.777). On comparing the 3 groups on disease-free survival, there was insignificant differences among the three AFP classes (χ2=1.859, P=0.3975) as shown in Figure 2.
The Kaplan-Meier overall survival curves of the 153 studied patients, subdivided according to their Child class (Child A and Child B) at the time of diagnosis of HCC, revealed that Child class A patients showed a better survival after RFA than those with class B (χ2=34.613, P=0.000).

Figure 1 Kaplan-Meier survival curves showing the overall survival of the 153 studied patients subdivided according to their alpha-fetoprotein serum levels at the diagnosis of HCC (<20ng/ml; 20-200ng/ml; >200ng/ml).
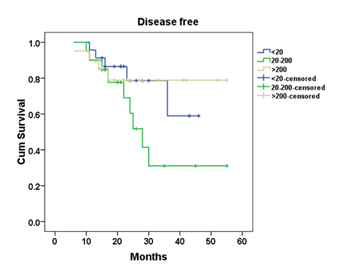
Figure 2 Kaplan-Meier survival curves showing the disease-free survival of the 153 studied patients subdivided according to their alpha-fetoprotein serum levels at the diagnosis of HCC (<20ng/ml; 20-200ng/ml; >200ng/ml).
The Kaplan-Meier overall survival curves of the studied patients, subdivided according to their lesion size at the time of HCC diagnosis (<3 and >/=3cm), revealed no statistically significant differences (χ2=0.305, P=0.581). Similarly, comparison of the patients in their lesion number at the time of diagnosis of HCC (uni-nodular and multi-nodular), the Kaplan-Meier survival curves showed insignificant differences (χ2=0.001, P=0.979).
Alpha-fetoprotein had an inadequate accuracy in discriminating survivors and deceased patients (AUC0.435, 95% CI 0.338-0.531) (Figure 3). Also, AFP had an inadequate accuracy to discriminate patients with recurrence from those without recurrence (AUC=0.476, 95% CI 0.378-0.573) (Figure 4).
Several studies have reported the ability of AFP response to predict response to therapy and survival outcomes.7 However, there is no consensus yet regarding the magnitude of the decrease in AFP that defines AFP response.8
Among cases treated with RFA, HCC recurred in 88cases (57.5%) in the current study. Recurrence of HCC after RFA is neither uncommon nor specific to this therapy. Even patients treated with hepatic resection showed recurrence rates >70% 5years after surgery.9
A large study demonstrated that the etiology of liver disease is an important predictor of long-term survival and distant intrahepatic recurrence after RFA and identified a role for chronic hepatitis C virus (HCV) in survival.10 In another study, it was shown that patients with HCV-related cirrhosis who achieved sustained hierological response to antiviral therapy have a substantially lower rate of HCC recurrence and accompanying higher survival rate.11 More than 90% of patients in the current study were HCV-positive and this may have played a role in the high recurrence rate.
In the present study, regarding overall survival, Child class A patients could achieve a better survival after RFA than those with Child class B (χ2=34.613, P=0.000). Similar to our results, Lee et al.,12 found that Child-Pugh class B (relative risk=2.43, P=.011) is one of the significant predictive factors for poor overall survival. Similarly, Kikuchi et al.,13 found that the survival of their patients was associated with the Child-Pugh class. Because most HCCs arise in the context of liver cirrhosis, the established liver dysfunction may already represent generally a poor prognosis.3
In the present study, Kaplan-Meier overall survival curves of the studied patients, who were subdivided according to their lesion size at the time of diagnosis of HCC (<3 and >/=3cm), revealed no statistically significant difference (χ2=0.305, P=0.581). This finding is consistent with the results of Giannini et al.,14 who found that there was no significant survival difference associated with the size of the HCC (≤ 2 or 2-3cm).
In the present study, the survival rate at 5years was 48.8%, while in the study by Giannini et al.,14 the 5-year survival rate was approximately 60% in both patients with AFP serum levels below and above 200ng/ml. The higher survival rate in the study of Giannini et al.,14 comes from the different treatment modalities. In their study, they used all curative modalities including orthotropic liver transplantation, hepatic resection, percutaneous ethanol injection and RFA. In our study, all our patients were treated with RFA only.
It seems that the predictive ability of AFP depends mainly on tumor size and treatment modality, being more evident in patients with advanced HCC and in those who received a palliative treatment, and less evident in patients with small tumors and in those who underwent curative treatment.15
Indeed, the prognostic role of AFP was dramatically diluted in studies excluding patients with advanced liver disease and/or advanced HCC.16 These considerations are also supported by the evidence in our series that there was no ‘‘therapeutic disparity,’’ and that causes of death were evenly distributed across patients with normal, mildly, and markedly elevated AFP levels, likely ruling out the presence of other possible prognostic confounding factors.
Overall survival (time to death or end of the study in months), was 95%, 75.6%, 55.6%, 48.8%, and 48.8% at 1, 2, 3, 4, and 5years respectively. The mean survival interval was 33.6months in group 1, 34.3months in group 2, and 28.6months in group 3 with no significant differences among the three groups (p=0.207).
In the study by Farinati et al.,17 the mean survival time for the group of treated patients with AFP levels (<20ng/ml) was 39months, while it was 31months and 20months for the patients with AFP levels of (21- 400ng/ml) and (>400ng/ml), respectively, with a significant link between AFP level and survival time.
This difference could be attributed to several factors, such as the larger study population in the study of Farinati et al.17 Additionally, their study population was not homogenously distributed among the three groups as patients were subdivided into 3 AFP groups: normal (<20ng/ml) [46% of the study population], elevated (21– 400 ng/ml) [36% of the study population], and diagnostic (> 400ng/ml) [18% of the study population].
In the present study, the ROC curve showed that AFP had inadequate accuracy in discriminating survivors and deceased patients (AUC 0.435, 95%CI=0.338-0.531). These results are consistent with that of Giannini et al.14 (AUC 0.536, 95% CI=0.465-0.606).
From our results, we demonstrated that AFP level could not be used as a good predictor of either death or recurrence. This finding agrees with the study by Kiriyama et al.,18 who found that serum AFP levels did not have value in predicting recurrence or death.
Contrary to our results, a recent study by Zhang et al.,19 concluded that tumor size, albumin, prothrombin time, and α- fetoprotein levels were independently associated with mortality after RFA for HCC, while tumor size and HBV-DNA were independently associated with recurrence.
The difference between our results and those of Zhang et al.,19 can be attributed to many factors. First, they included only patients with high AFP before treatment, while we included all patients with different levels. Second, the viral status of their patients was HBV, while in our study; approximately 93% of our patients were HCV-positive. The prognostic role of alpha-fetoprotein reported in other studies may be due to the heterogeneous liver and tumor-related characteristics, as well as different modalities of HCC treatment in the studied populations.9
A major limitation of the current study is that it is a retrospective study with a relatively small number of patients. Furthermore, the feasibility of RFA is mainly dependent on the operator’s technique, the experience, and the equipment available at the center. Moreover, the findings in the current study were obtained from a single-center cohort and cannot be compared to clinical experience at other treatment centers, due to the heterogeneity of selection and patient management, physician expertise, the indication for additional treatments, and the institution’s volume of care. In conclusion, our results demonstrated that AFP level could not be used as a good predictor of either death or recurrence after RFA in HCC cases.
None.
The author declares no conflict of interest.
This study involved human participants and was reviewed and approved by the Ethics Committee of Ain Shams University Hospitals.
Patients were not required to give informed consent to the study because the records of patients were reviewed in a retrograde manner and the clinical data that were obtained after each patient agreed to treatment by written consent.

©2018 Khalifa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.