eISSN: 2373-6372


Research Article Volume 7 Issue 1
1Department of Pathology, Hospital of the Order of the Brothers of Saint John of God in Budapest, Hungary
2Department of Rheumatology, St. Margaret Clinic Budapest, Hungary
Correspondence: Miklós Bély, Department of Pathology, Hospital of the Order of the Brothers of Saint John of God in Budapest, Hungary
Received: April 15, 2017 | Published: June 16, 2017
Citation: Bély M, Apáthy A (2017) Prevalence and Mortality of Gastric and Duodenal Ulcers in Rheumatoid Arthritis - A Retrospective Clinicopathologic Study of 234 Autopsy Patients. Gastroenterol Hepatol Open Access 7(1): 00221 DOI: 10.15406/ghoa.2017.07.00221
Aim: The aim of this study was to determine the prevalence and mortality of gastric (gU) and duodenal (dU) peptic ulcers in rheumatoid arthritis (RA). Also, to evaluate the possible relationship between gU and dU and autoimmune vasculitis (A-SV), AA amyloidosis (AAa) or lethal septic infection (SI) with or without purulent arthritis (PA).
Patients and methods: A randomized autopsy population of 234 in-patients with RA was studied. RA was confirmed clinically according to the criteria of the ACR. The presence of gU and dU, A-SV, AAa, SI, or PA was determined at autopsy and supported by histological examination. The relationships between prevalence and mortality of gU or dU and A-SV, AAa, SI or PA were analyzed by Pearson's chi-squared (χ2) test.
Results and conclusions: gU was found in 11 (4.70%), dU in 9 (3.85%), A-SV in 47 (20.08%), AAa in 48 (20.51%), and SI in 31 (13.24%), accompanied with PA in 15 (6.41%) of 234 patients. The negative correlation between A-SV, AAa, or PA and prevalence of gU or dU suggests that-in our autopsy population-A-SV, AAa or PA had no pathogenic role in development of gastric or duodenal ulcers. A-SV, AAa or PA not influenced the mortality of gU or dU. Gastric or duodenal ulcers can be regarded as associated diseases of RA and not as complications of it. The significant connection between SI and prevalence and mortality of gU or dU indicates a causal relationship between them: the development of gU or dUs increase the risk of lethal SI.
Keywords: rheumatoid arthritis, gastric and duodenal peptic ulcers, autoimmune vasculitis, AA amyloidosis, lethal septic infection; purulent arthritis
Gastric and duodenal peptic ulcers (gU or dU) are of different etiologies and are common in rheumatoid arthritis (RA). The prevalence of gU or dU is higher in RA than in the general population.1 Numerous early and recent studies discuss the relationship between gU or dU and non-steroidal anti-inflammatory drugs,2-5 corticosteroids,4-8 anticoagulants,6 with or without other contributing common factors (sex, age, smoking, alcohol consumption, etc.).The therapy induced peptic ulcers (with complications like perforations, peritonitis, septic infections or bleeding) play an important role in the mortality of RA.9,10 Gastrointestinal involvement by complications of RA, such as systemic vasculitis or amyloidosis - with or without peptic ulcers-is also well known.9-11 The aim of this study was to determine the prevalence and mortality of gU and dU in RA. Also, to identify the possible role of systemic autoimmune vasculitis (A-SV) or AA amyloidosis (AAa) in the prevalence and mortality of gU and dU, furthermore to evaluate the possible relationship between gU and dU and lethal septic infection (SI) with or without purulent arthritis (PA).
Patients and methods
At the National Institute of Rheumatology 11558 patients died between 1969 and 1998; among them 234 with RA (females 170, average age: 66.31 years, range 88-16, onset of RA: 50.46, average disease duration: 12.96 years; males 64, average age: 66.08 years, range 88-19, onset of RA: 52.55, average disease duration: 12.96 years at death), and all of them were autopsied.
RA was confirmed clinically according to the criteria of the American College of Rheumatology (ACR).12 The basic disease, its complication(s), and the lethal outcome caused by gU and dU were determined and analyzed retrospectively, reviewing the clinical and pathological reports. The presence of gU or dU and A-SV, AAa furthermore SI (with or without PA) was determined at autopsy and confirmed by a detailed review of extensive histological material. From each patient 50-100 tissue blocks of 12 organs (heart, lung, liver, spleen, kidneys, pancreas, gastrointestinal tract, adrenal glands, skeletal muscle, peripheral nerve, skin and brain) were studied microscopically.13 Amyloid A deposits were diagnosed histologically according to Romhányi14 by a modified (more sensitive) Congo red staining,15 and were confirmed histochemically.16 The relationships between prevalence and mortality of gU or dU and A-SV, AAa furthermore SI (with or without PA) were analyzed by Pearson's chi-squared (χ2) test.17
gU was found in 11 (4.70%), and dU in 9 (3.85%) of 234 RA patients (Figure 1.1). Seven gU of 11, and 6 dU of 9 led to death in 13 (65.0 %) of 20 patients (Figure 1.2). In 4 patients (in 2 with gU and in 2 with dU) the direct cause of death was massive internal bleeding, and in 3 (in 2 with gU and in 1 with dU) it was perforation of an ulcer accompanied by peritonitis. Perforated gU (n=3 of 11) and dU (n=3 of 9) existed in further 6 patients with peritonitis and generalized lethal SI (the outcome of 3 gU and 3 dU in all of these 6 cases were lethal). In 7 (35.0 %) of 20 patients 4 gU and 3 dU existed without lethal complications (Figure 1.2). gU and dU (with or without lethal outcome) were recognized clinically in 14 (in 8 with gU and in 6 with dU; 70.0 %) of 20 cases, and missed in 6 (in 3 with gU and in 3 with dU; 30.0 %) of 20 (Figure 1.3). A-SV (Figure 2a-d) was observed in in 47 (20.08%), AAa (Figure 3 & 4) in 48 (20.51%), and SI in 31 (13.24%), accompanied with PA in 15 (6.41%) of 234 patients.
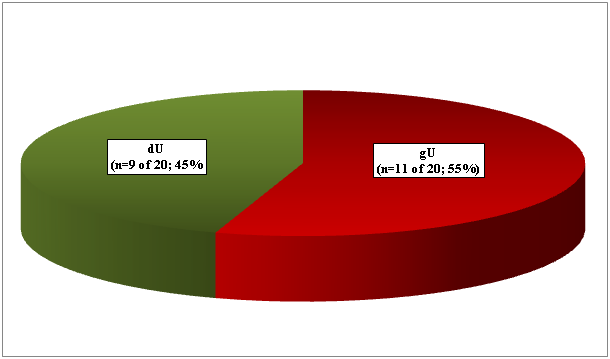
Figure 1.1 Prevalence of gastric n=11 (55.0%) and duodenal n=9 (45.0%) peptic ulcers in 234 RA patients.
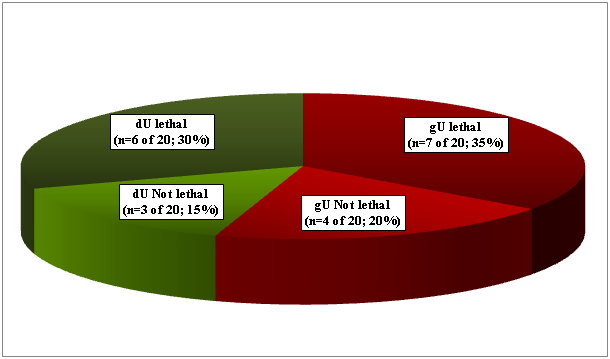
Figure 1.2 Prevalence: n=20 (8.55%), and mortality n=13 (5.56%) of gastric or duodenal peptic ulcers-by bleeding, peritonitis or SI- in 234 RA patients.
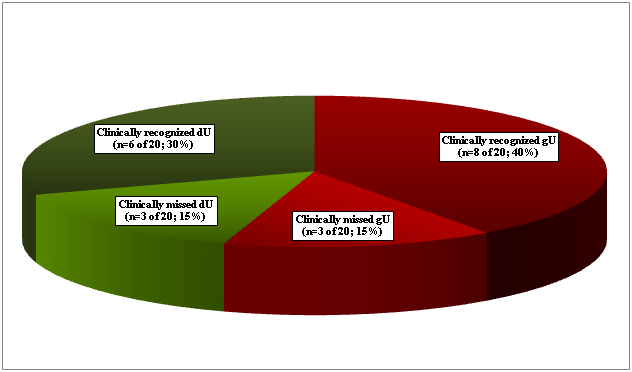
Figure 1.3 Clinically recognized n=14 (70.0%) or missed n=6 (30.0%) of gastric or duodenal peptic ulcers in 234 RA patients.
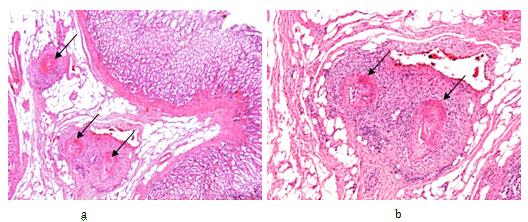
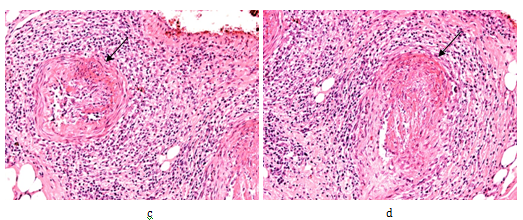
Figure 2 Rheumatoid arthritis, gastrointestinal tract (stomach), systemic vasculitis of autoimmune origin (A-SV).
Small arteries, non-specific vasculitis with sectorial fibrinoid necrosis (arrows) in subacute-subchronic stage of inflammation.
(a) HE, x 20, (b) same as (a) x40, (c) and (d) same as (a) x100
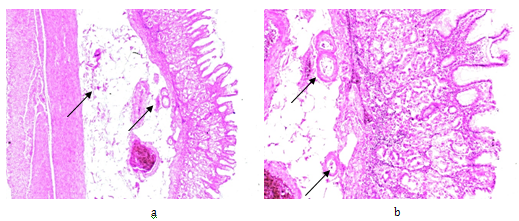
Figure 3 Rheumatoid arthritis, gastrointestinal tract (stomach), systemic secondary AA amyloidosis (AAa).
Amyloid A deposits in the wall of arterioles, small arteries and within interstitial reticulin and collagen fibers (arrows).
(a) PAS, x 50, (b) same as (a) x125
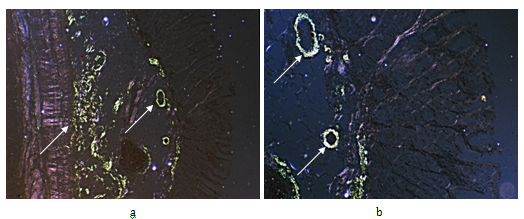
Figure 4 Rheumatoid arthritis, gastrointestinal tract (stomach), systemic secondary AA amyloidosis (AAa).
Same as Figure 3ab, Congo red staining, without alcoholic differentiation, covered with gum arabic. Viewed under polarized light (a) x50 (b) same as (a) x125
Sex, average age (range) and disease duration, and onset of RA in patients with or without gU or dU, and with or without A-SV, AAa, SI, or PA are summarized in Table 1. The basic disease, complication(s) and associated diseases of 20 RA patients with gU or dU are summarized in Table 2. gU or dU were associated with A-SV in 3, with AAa in 3, with lethal SI in 6 of 20 patients. gU or dU with lethal outcome were associated with systemic A-SV in 3, with AAa in 3, with lethal SI in 6 of 13 patients. In this autopsy population gU or dU was never associated with PA.
Sex |
Number of Autopsies |
Average age in years at Death |
Range (in Years) |
Age at Onset of Disease |
Disease Duration (in Years) |
RA patients |
234 |
66.25 |
88 - 16 |
51.02 |
14.76 |
Female |
170 |
66.31 |
88 - 16 |
50.46 |
15.42 |
Male |
64 |
66.08 |
88 - 19 |
52.55 |
12.96 |
With gU or dU |
20 |
65.1 |
88 - 45 |
51.15 |
13.31 |
Female |
14 |
66 |
88 - 45 |
51.44 |
12.33 |
Male |
6 |
63 |
70 - 48 |
50.5 |
15.5 |
Without gU or dU |
214 |
66.36 |
88 - 16 |
51.01 |
14.87 |
Female |
156 |
66.34 |
88 - 16 |
50.39 |
15.65 |
Male |
58 |
66.4 |
88 - 19 |
52.73 |
12.73 |
A-SV |
47 |
68.09 |
88- 32 |
55.89 |
12.8 |
Female |
29 |
68.83 |
88- 32 |
55.69 |
14.23 |
Male |
18 |
66.89 |
83- 53 |
56.17 |
10.72 |
Without A-SV |
187 |
65.78 |
88 - 16 |
49.46 |
15.38 |
Female |
141 |
65.79 |
88 - 16 |
48.92 |
15.8 |
Male |
46 |
65.76 |
88 - 19 |
51.25 |
14 |
With AAa |
48 |
63.75 |
88 - 19 |
46.66 |
17.18 |
Female |
38 |
65.13 |
88 - 32 |
46.54 |
17.91 |
Male |
10 |
58.5 |
88 - 19 |
47.11 |
14.33 |
Without AAa |
186 |
66.9 |
88 - 16 |
52.41 |
13.99 |
Female |
132 |
66.66 |
88 - 16 |
51.65 |
14.56 |
Male |
54 |
67.5 |
87 - 20 |
54.22 |
12.63 |
With SI |
31 |
62.45 |
83 - 41 |
49.59 |
13 |
Female |
22 |
61.5 |
83- 41 |
49.53 |
12.32 |
Male |
9 |
64.78 |
71 - 52 |
49.75 |
14.63 |
SI with PA |
15 |
59.47 |
71 - 46 |
44.08 |
16.38 |
Female |
10 |
58.2 |
68 - 46 |
42.88 |
16.63 |
Male |
5 |
62 |
57 - 20 |
46 |
16 |
SI without PA |
16 |
65.25 |
83 - 41 |
54.71 |
9.86 |
Female |
12 |
64.25 |
83 - 41 |
54.36 |
9.18 |
Male |
4 |
68,3 |
70 - 66 |
56 |
12.33 |
Without SI |
203 |
66,1 |
88 - 16 |
51,1 |
14,9 |
Female |
148 |
66,1 |
88 - 16 |
49,9 |
15,9 |
Male |
55 |
66,3 |
87 - 19 |
54,1 |
12,5 |
Without Complications |
121 |
68.01 |
88 - 16 |
51.77 |
15.1 |
Female |
92 |
67.59 |
88 - 16 |
50.44 |
15.56 |
Male |
29 |
70,3 |
87 - 20 |
56.28 |
13.56 |
Table 1 Sex, average age (range), onset of disease and disease duration (in years) of RA patients with or without gU or dU and SV, AAa, SI or PA
Basic Disease |
Complication (1-2) |
Complication (3) |
Cause of Death |
Associated Disease(s) |
Cl+ Cl- |
Protocol n/Year |
||
1 |
RA |
Gastric ulcer1-Perforation |
Gastrectomy |
Bronchopneumonia |
Ath-DM |
Cl* |
278/71 |
|
2 |
RA |
Gastric ulcer2-Colitis |
Perforation |
Peritonitis- Lethal SI1 |
Ath-DM |
Cl- |
228/72 |
|
3 |
RA |
Felty Syndrome-Splenectomy |
Duodenal ulcer1 |
Massive internal bleeding1 |
Liver necrosis (red) |
Cl* |
320/72 |
|
4 |
Ath |
Thrombosis of femoral vein |
Gastric ulcer3 |
Pulmonary embolism |
RA-DM5 |
Cl* |
51/74 |
|
5 |
RA |
Gastric ulcer4-Bleeding |
Gastrectomie |
Circulatory failure |
Ath |
Cl* |
334/75 |
|
6 |
RA |
Nephritis |
Gastritis and Duodenal ulc2 |
Uraemia- Bleeding2 |
Cl* |
197/76 |
||
7 |
Ath |
Myocardial fibrosis |
Duodenal ulcer3 |
Purulent bronchitis and bronchiolitis |
RA-TbF-Meningeoma |
Cl- |
318/76 |
|
8 |
Ath |
Myocardial fibrosis |
Gastric ulcer5 |
Circulatory failure |
RA |
Cl* |
386/76 |
|
9 |
RA |
AA1 |
Gastric ulcer6-Perforation |
Peritonitis |
Lethal SI2 |
Cl- |
162/78 |
|
10 |
RA |
Duodenal ulcer4-Colitis |
Lethal SI3 |
Cl- |
243/78 |
|||
11 |
RA |
Erosive gastritis7 |
Massive internal bleeding3 |
Ath |
Cl* |
327/78 |
||
12 |
RA |
Duodenal ulcer5 |
Circulatory failure |
DM-Hepatic cirrhosis |
Cl* |
385/78 |
||
13 |
RA |
AA2 |
Duodenal ulcer6-Perforation |
Peritonitis |
Cl* |
76/79 |
||
14 |
RA |
Ulcerative gastritis8-Colitis |
Diverticulitis of Colon-Perforation |
Lethal SI4 |
Cl- |
55/82 |
||
15 |
RA |
SV1 |
Duodenal ulcer7-Perforation |
Peritonitis |
Lethal SI5 |
Ath |
Cl- |
318/89 |
16 |
RA |
Gastric ulcer9 |
Perforation- |
Peritonitis (local) - Circulatory failure |
JCA |
Cl* |
25/90 |
|
RA |
Gastric ulcer10 |
Perforation |
Peritonitis |
Cl* |
205/91 |
|||
18 |
RA |
Gastric ulcer11- Perforation |
Atlantoaxial subluxation |
Peritonitis |
HT |
Cl* |
208/93 |
|
19 |
RA |
SV2 |
Duodenal ulcer8-Perforation |
Lethal SI5 |
TbFc |
Cl* |
375/95 |
|
20 |
RA |
SV3 |
AA2 |
Duodenal ulcer9 |
Massive internal bleeding4 |
Cl* |
33/96 |
|
Table 2 Prevalence and mortality of gastric or duodenal peptic ulcers in 20 of 234 RA patients
The relationship between gU or dU and coexistent SV, AAa, furthermore lethal SI (with or without PA) in 234 RA patients is summarized in Table 3. There was no significant correlation between A-SV, AAa, PA and prevalence or mortality of gU or dU. There was a significant correlation between SI and prevalence and mortality of gU or dU. A-SV coexisted with AAa in 9 of 47 patients. The relationship between A-SV and AAa was not significant (x²=0.0032, p<0.95); the A-SV did not influence the prevalence of AAa, and AAa did lead to A-SV in our autopsy population.
Prevalence of Complications in 234 RA pts. |
Prevalence of gU or dU |
Mortality of gU or dU |
A-SV n=47 |
χ²=0.09107*, p<0.76 |
χ²=0.0062, p<0.93 |
AAa n=48 |
χ²=0.1217*, p<0.72 |
χ²=0.0138, p<0.91 |
Lethal septic infection n=31 |
χ²=5.3400, p<0.02 |
χ²=12.9685, p<0.0003 |
Purulent arthritis n=15 |
χ²=0.5573*, p<0.45 |
χ²=0.1508*, p<0.69 |
Table 3 Relationship between prevalence and mortality of gU, or dU and coexistent SV, AAa, furthermore SI (with or without PA) in 234 RA patients (p<0.05)
Numerous publications discuss the prevalence of peptic ulcers (Table 4), vasculitis (Table 5), amyloidosis (Table 6) or lethal septic infection (Table 7) in RA with or without its role in mortality.17-55 Unfortunately these studies do not specify the relationship between gU or dU and A-SV, AAa or SI. In most of these early studies the prevalence or mortality of gU or dU seems to be underestimated, presumably due to the limited microscopic examination of the gastrointestinal tract.
Authors |
Reference Year of Publication |
Autopsy |
Prevalence of gU or dU |
Mortality of gU or dU |
Baggenstoss and Rosenberg |
1943 [17] |
30 |
ND |
2* - 6.66% |
Rosenberg and Baggenstoss |
1943 [18] |
30 |
ND |
2* - 6.66% |
Young and Schwedel |
1944 [19] |
33 |
0 - 0% |
0 - 0% |
Teilum and Lindahl |
1954 [20] |
28 |
0 - 0% |
0 - 0% |
Gedda |
1955 [21] |
45 |
ND |
0 - 0% |
Sinclair and Cruickshank |
1956 [22] |
16 |
4** - 25.0% |
0 - 0% |
Leboowitz |
1963 [23] |
62 |
1 - 1.61% |
1 - 1.61% |
Ozdemir et al. |
1971 [24] |
47 |
16 - 34.04% |
ND |
Püschel |
1973 [25] |
143 |
28 - 19.58% |
13 - 9.09% |
Eulderink |
1976 [26] |
111 |
ND |
0 - 0% |
Suzuki et al. |
1994 [27] |
81 |
ND |
2 - 2.47% |
Bély and Apáthy |
2007 [28] |
161 |
14 - 8.69% |
9 - 5.59% |
Bély and Apáthy |
2017 [29] |
234 |
20 - 8.54% |
13 - 5.55% |
Table 4 Prevalence and mortality of peptic ulcers in autopsy material of rheumatoid arthritis
Remarks to Table 4
ND No Data
*mentioned as gastrointestinal diseases
**caused by marked intestinal amyloidosis
Footnote to Table 4
* Negative value of association’s coefficient; Significant value is in bold
Authors |
Year of Publication, References |
Autopsy |
Prevalence of Vasculitis |
Mortality of Vasculitis |
Cruickshank |
1954 [30] |
72 |
18 - 25% |
ND |
Sinclair and Cruickshank |
1956 [22] |
16 |
9 - 56.3% |
ND |
Cruickshank* |
1958 [31] |
100 |
20* - 20% |
ND |
Lebowitz |
1963 [23] |
62 |
6 - 10% |
ND |
Sokoloff |
1964 [32] |
19 |
2 - 10.5% |
ND |
Karten** |
1969 [33] |
102 |
6** - 6% |
ND |
Gardner |
1972 [34] |
142 |
7 - 4.9% |
ND |
Davis and Engleman |
1974 [35] |
62 |
6 - 10% |
ND |
Eulderink |
1976 [26] |
111 |
ND |
7 - 6.3% |
Albada-Kuipers et al. |
1986 [36] |
173 |
17 - 10% |
ND |
Boers et al. |
1987 [37] |
132 |
18 - 13.6% |
ND |
Suzuki et al. |
1994 [27] |
81 |
25 - 30.8% |
ND |
Bély and Apáthy*** |
1993 [38] |
161 |
36 - 22.4% |
19 - 11.8% |
Bély and Apáthy*** |
2006 [39] |
234 |
51 - 21.8 |
23 - 9.8% |
Table 5 Prevalence and mortality of systemic vasculitis (SV) at autopsy of RA patients (no mention of the origin of SV)
Remarks to Table 5
ND No Data
*Coronaritis
**102 patients with RA-partially autopsied (Karten)
***The studies discuss 36 SV-33 of autoimmune origin and 3 of septic origin.
****The studies discuss 51 SV- 47 of autoimmune origin and 4 of septic origin; the latter 4 SV of septic origin have been excluded in the present study.
Authors |
Year of Publication References |
Autopsy |
Prevalence of Amyloidosis |
Mortality of Amyloidosis |
Bayles |
1943 [40] |
23 |
ND* |
3 - 13.0% |
Baggenstoss and Rosenberg |
1943 [17] |
30 |
2 - 6.6% |
1 - 3.3% |
Young and Schwedel |
1944 [19] |
33 |
5 - 15.2% |
0 - 0% |
Unger et al. |
1948 [41] |
58 |
4 - 6.9% |
ND |
Teilum and Lindahl |
1954 [20] |
28 |
17 - 60.7% |
7 - 25.0% |
Gedda |
1955 [21] |
45 |
11 - 24.4% |
9 - 20.0% |
Sinclair and Cruickshank |
1956 [22] |
16 |
4 - 25.0% |
0 - 0% |
Missen and Tailor |
1956 [42] |
47 |
8 - 17.0% |
4 - 8.5% |
Leboowitz |
1963 [23] |
62 |
6 - 10.0% |
ND |
Sokoloff |
1964 [32] |
19 |
0 - 0% |
0 - 0% |
Cohen |
1968 [43] |
42 |
11 - 26% |
ND |
Karten |
1969 [33] |
95 |
1 - 1.05% |
ND |
Gritsman |
1969 [44] |
15 |
6 - 40.0% |
ND |
Ozdemir et al. |
1971 [24] |
47 |
1 - 2.1% |
ND |
Gardner |
1972 [34] |
142 |
17 - 11.97% |
ND |
Püschel |
1973 [25] |
143 |
15 - 10.5% |
ND |
Vroninks et al. |
1973 [45] |
62 |
3 - 4.84% |
0 62- 0% |
Hajzok et al. |
1976 [46] |
16 |
7 - 43.7% |
ND |
Eulderink |
1976 [26] |
111 |
ND |
6 111- 5.4% |
Rainer et al. |
1978 [47] |
79 |
ND |
4 79- 5.0% |
Boers et al. |
1987 [48] |
132 |
14 - 10.6% |
ND |
Bély |
1991 [49] |
161 |
34 - 21.1% |
17 - 11% |
Suzuki et al. |
1994 [27] |
81 |
17 - 21.0% |
6 - 7.4% |
Bély and Apáthy |
2006 [39] |
234 |
48 - 20.5% |
20 - 8.5%% |
Table 6 Prevalence and mortality of AA amyloidosis in autopsy material of rheumatoid arthritis (identified amyloid deposits by different staining methods, such as: Toluidine blue, Crystal violet, Syrius red, Congo-red staining according to Romhányi, Bennhold’s, Puchtler’s, Bély’s Congo red method)
Remarks to Table 6
ND No Data
Authors |
Reference, Year of Publication |
Autopsy |
Clinically recognized SI |
Mortality of sepsis |
Bayles* |
1943 [40] |
23 |
ND |
2 - 8.7% |
4 - 17.4% |
||||
Young and Schwedel |
1944 [19] |
33 |
ND |
1 - 3.03% |
Bywaters* |
1950 [50] |
27 |
ND |
1 - 3.7% |
Gedda |
1955 [21] |
45 |
ND |
8 - 18% |
Lebowitz |
1963 [23] |
62 |
ND |
2 - 3.2% |
Bonfiglio and Atwater |
1969 [51] |
47 |
ND |
4 - 8.5% |
Gardner |
1972 [34] |
142 |
ND |
8 - 5.6% |
With septic arthritis |
5- 3.5% |
|||
Without septic arthritis |
3 - 2.1% |
|||
Russel and Ansell |
1972 [52] |
17 |
ND |
2 - 11.7% |
Püschel |
1972 [25] |
143 |
9 - 6% |
8 - 5.6% |
Vroninks and mtsi. |
1973 [45] |
62 |
2 - 3.2% |
3 - 4.8% |
Eulderink** |
1976 [26] |
111 |
ND |
3 - 2.7% |
Rainer et al. |
1978 [47] |
79 |
ND |
21 - 27% |
Reilly et al. |
1990 [53] |
63 |
ND |
3 - 4.8% |
Bély et al.** |
1992 [54] |
100 |
ND |
10 - 10% |
Toyoshima et al.**** |
1993 [55] |
1246 |
ND |
52 - 4.2% |
Suzuki et al. |
1994 [27] |
81 |
ND |
5 - 6.2% |
Bély*** |
present work |
161 |
11 - 6.83% |
24 - 14.9% |
With prulent arthritis |
6 - 3.7 |
12 - 7.45% |
||
Without prulent arthritis |
5 - 3.1% |
12 - 7.45% |
||
Table 7 Prevalence of septic infection in autopsy material of rheumatoid arthritis
Remarks to Table 7
ND No Data
*Endocarditic bacterial infection (1), or lethal purulent peritonitis (3) leading to death
**Lethal septic infection accompanied with purulent arthritis only
***All lethal septic infections with or without purulent arthritis
****Based on national mortality statistics of Japan without detailed histological analysis of organ involvement.
It is difficult to estimate the true prevalence of A-SV, AAa or SI. Most studies do not specify the type of vasculitis (autoimmune, septic-bacterial, viral, fungal-hypertonic, endocrine disease associated, etc.). Amyloidosis in most studies is diagnosed with different methods of diverse specificities and sensitivities (with or without identification of the types of amyloid deposits).
According to our best knowledge a detailed analysis of A-SV or AAa and its role in the prevalence and mortality of gU or dU has not been available in the literature. The negative correlation between A-SV (c²=0.09107, p<0.76), AAa (c²=0.1217, p<0.72) or PA (c²=0.5573, p<0.45), and prevalence of gU or dU suggests that-in our autopsy population - A-SV, AAa or PA had no pathogenic role in the development of gastric or duodenal ulcers.
A-SV (x²=0.0062, p<0.93), AAa a (x²=0.0138, p<0.91) or PA (c²=0.1508, p<0.69) did not influence the mortality of gU or dU. Gastric or duodenal ulcers may be regarded as associated diseases of RA and not as complications. The significant correlation between gU or dU and lethal SI indicates a causal relationship: the development of gU or dUs increases the risk of lethal SI; in case of a perforated ulcer there is a direct relationship. The positive and significant correlation between SI and PA-as it was found in our previous study13 -indicates also a similar causal relationship. At the same time the negative association’s coefficient (-1) and lack of a significant correlation between gU or dU and PA suggests that these are individual phenomena which may exist simultaneously in RA leading to lethal SI independently from each other.
Vasculitis or amyloidosis may cause gastrointestinal complaints, diarrhea, erosions, and hemorrhages, even peptic ulcers with bleeding, perforation, peritonitis or lethal sepsis. Detailed histological study of a large autopsy population of RA supports and statistically confirms that gU or dU are independent phenomena in RA and their prevalence and mortality is not influenced by the leading complications of RA, e.g by A-SV or AAa. (Peptic ulcers - in agreement with the literature-are probably related to the therapy of RA). gU or dU significantly increases the risk of lethal septic infections, but are independent of purulent arthritis (based on our previous study PA should be regarded a specific source of SI13).
None.
Authors declare that there is no conflict of interest.

©2017 Bély, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.