eISSN: 2373-6372


Review Article Volume 14 Issue 4
1Department of Physiology, Adeleke University, Nigeria
2Department of Physiology, Babcock University, Nigeria
3Department of Physiology, Babcock University, Nigeria
4Department of Physiology, University of Ibadan, Nigeria
Correspondence: Oyovwi O. Mega, Department of Physiology, Adeleke University, Ede, Osun State, Nigeria , Tel +2348066096369
Received: April 10, 2023 | Published: July 17, 2023
Citation: Mega OO, Ojetola AA, Oghenetega OB, et al. Physiological modulation of adipogenic transcription factors: A possible therapeutic target for the prevention of metabolic pathologies-related oxido-inflammatory responses. Gastroenterol Hepatol Open Access. 2023;14(4):105-111. DOI: 10.15406/ghoa.2023.14.00554
Atherosclerosis and its complications, fatty liver diseases, insulin resistance (IR), type 2 diabetes, osteoarthritis, rheumatoid arthritis, and cancer are just a few of the age-related oxido-inflammatory responses that are predisposed by metabolic pathologies brought on by excess adiposity. Intriguingly, adipose tissue is an organ capable of mediating biological effects on inflammation and metabolism, which aids the maintenance of energy homeostasis. These reviews highlight the importance of understanding the metabolic and oxido-inflammatory pathways and homeostasis, and the molecules that are essential to the metabolic and pathogenic signaling sensor pathway systems. This may be helpful to understand the mechanisms and potential treatment options that will boost the endogenous molecules for prevention of stress and inflammatory conditions induced through metabolic signaling. In conclusion, this review study shows that problems caused by poor metabolic homeostasis may be successfully resolved. Hence, in the future, different adipocytokines route that mediated control of oxido-inflammation via modulation of ICDH, PPAR- γ, NR4A1, ChREBP and SREBP-1c will be highly effective for preventing metabolic disease.
In this review, we attempt to summarize our current understanding of the Physiological role of adipogenic transcription factors in metabolic pathologies-related oxido-inflammatory responses, focusing on recent studies that explore the ICDH, PPAR- γ, NR4A1, SREBPs and ChREBP signaling pathways for the preventions of metabolic pathologies-related oxido-inflammatory responses.
Keywords: secreted frizzled-related protein, secreted frizzled-related protein, wingless-type MMTV integration site family member 5A, IL interleukin
SFRP5, secreted frizzled-related protein 5; FABP-4, fatty acid binding protein 4; ASP, acylation-stimulating protein; RBP4, retinol-binding protein 4; mTOR, mammalian target of rapamycin; IRS, insulin receptor substrate; Wnt5a, wingless-type MMTV, integration site family member 5A; AT, adipose tissue; AdipoR, adiponectin receptor; AMPK, AMP-activated protein kinase; PPAR, α peroxisome proliferator-activated receptor alpha; GSIS, glucose-stimulated insulin secretion; FABP-4, fatty acid binding protein 4; RBP4, retinol-binding protein 4; ASP, acylation-stimulating protein; LPS, lipopolysaccharides; IL, interleukin; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; C/EBP, CCAAT-enhancer-binding protein; TNF, tumor necrosis factor; NMN, nicotinamide mononucleotide; BMP4, bone morphogenetic protein 4
The digestive system is crucial in metabolic disorders.1 One of the most widespread and difficult diseases in contemporary medicine is metabolic disorder. The illnesses with the highest prevalence of metabolic failure include obesity, dyslipidemia, osteoporosis, diabetes mellitus, and metabolic syndrome. When physiological metabolic pathways are disturbed as a result of poor food, sedentary employment, and a lack of exercise, metabolic illnesses start to emerge. Metabolic illnesses can occur as a result of genetic variations in genes associated with glucose uptake and metabolism, insulin signalling or appetite regulation. Hence, insulin resistance, atherosclerosis, fatty liver disorders, diabetes, rheumatoid arthritis, osteoarthritis, and cancer are only a few of the age-related oxido-inflammatory response that are made more likely by an excess of body fat in metabolic disorders.2 The excess of adiposity results from an imbalance in the amount of energy consumed compared to how much is expended. The mounting evidence of obesity and the associated impairment of systemic metabolic homeostasis, as well as the induction of oxido-inflammatory flux, have enhanced the understanding of adipose tissue as a potential participant in regulating physio-pathological processes. Adipose tissue is currently assumed to have a role in the maintenance of energy balance as well as the development of metabolic and inflammatory problems related to obesity.3
This review examines the Physiological role of transcriptional factors associated adipokines in metabolic pathologies-related oxido-inflammatory responses.
Adipose tissue serves as the main source of fatty acids (FFA) for energy usage and heat production in the postprandial fasting state4. According to Unamuno et al.,5 adipose tissue, which is made up stromal vascular fraction and mature adipocytes where fibroblasts, blood cells, adipose-derived stem cells, and nerves reside, is an endocrine organ that plays a role in fat storage and release of bioactive polypeptides known as "adipokines" igure (Figure 1). These adipokines have the power to control blood pressure, inflammation, glucose homeostasis, body weight, and hunger.6
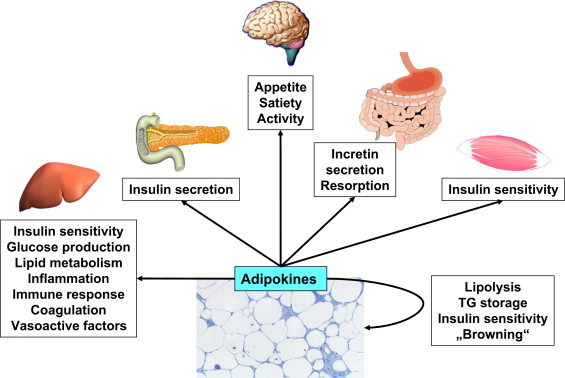
Figure 1 Shows how adipokines control vital physiological processes as blood pressure, hemostasis, inflammation, hunger, energy expenditure, and insulin sensitivity. They also have an autocrine and paracrine effect and regulate adipogenesis, adipose tissue lipolysis, and adipocyte metabolism.
Long-term metabolic stress causes inflammation, adipokine production, and adipose tissue malfunction which lead to liver injury as a result of increased lipid transport results in fatty liver disease. Liver and adipose tissue play important roles in body's overall energy homeostasis.7,8 Many bioactive adipokines secreted by adipocytes stimulate a number of cell signaling pathways in both central and peripheral organs. Obesity is connected to a number of illnesses and is linked to the altered synthesis of several adipokines. Additionally, adipokines plays a vital role in controlling insulin resistance, Barrett's esophagus, esophageal cancer, gastroesophageal reflux disease (GERD), colon polyps and cancer, viral hepatitis, gallbladder cancer, cholelithiasis, non-alcoholic fatty liver disease (NAFLD), and cholangiocarcinoma.9 Insulin resistance (IR) which normally develops prior to the onset of type 2 diabetes is known to be significantly influenced by the disease.10 Adipokines has an impact on tissues such as liver, muscles and adipose tissue.11 Adipose tissue releases cytokines that have a role in triggering and maintaining a proinflammatory state, which contributes to IR.12 These molecules also regulate the sensitivity and release of insulin13 Hence, the reduced adipokine production or dysregulation of adipogenic transcription factors found in obesity promotes diabetes etiology (Figure 2). Adipocytes release substances (such as free fatty acids and proinflammatory cytokines) that inhibit insulin-mediated glucose absorption in people with metabolic syndrome (MS).13
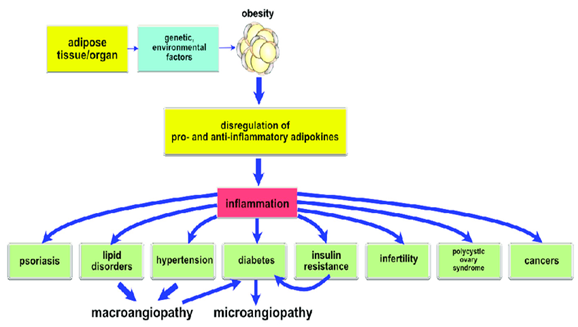
Figure 2 Showing the disregulation pathway of adipokines mediated metabolic disorders and chronic complication.13
Based on these findings as illustrated in Figure 2, IR and obesity are the factors responsible for the pathogenesis of metabolic disorders while proinflammatory condition also contributes to its development13. Visceral obesity has also been demonstrated to activate the majority of the pathways involved in metabolic disorders, highlighting the significance of a high caloric intake as a key causal component. All the proposed routes seem to point to chronic inflammation, IR, transcriptional factor changes, and neurohormonal activation as the main players in the progression of metabolic disease to cardiovascular diseases.
Adipokines are widely classified into two (2) types. These includes; (1) anti-inflammatory and (2) inflammatory adipokines. These classifications are based on their levels of expression in obese and type 2 diabetes mellitus patients. Adipokines which are upregulated in obese individuals are classified as inflammatory adipokines (Figure 3), and they usually exacerbate inflammation, insulin resistance, and glucose/insulin metabolism in adipose tissues as well as other peripheral tissues such as the liver, muscle, pancreas, and blood vessels: for example, FABP, ASP, RBP4, and lipocalin-2 are associated with inflammation, obesity, and insulin resistance; whereas anti-inflammatory adipokines are downregulated in obese. Figures 3A and 3B revealed the summary of the various pathological alterations in adipokine expression and highlighted the relevance and clinical significance of adipokines as therapeutic targets for obesity and T2DM. More so, adipokines which inhibit inflammation, such as adiponectin, omentin-1, SFRP5, and cardiotrophin-1, increase energy metabolism in the liver, skeletal muscle, and pancreas, as well as in adipose tissue.
Inflammatory flux mediated pathogenesis of metabolic pathologies
The key pathogenic characteristic is inflammation, which is linked to a disruption of the body's metabolic equilibrium14 The ensuing chronic inflammatory flux that was subsequently seen in this instance is a major contributor to the emergence of IR in tissues,15,16 As previously reported through decreased liver interleukin 1beta (IL-1β), interleukin-6 (IL-6), and tumor necrotic factor-alpha (TNF-α), and increased interleukin-10 (IL-10) indicating an increased inflammatory response and a disturbed immune system, adipocytokines' suppressive effect on oxidative stress has been linked to anti-inflammatory effect.17 These findings support past research indicating that the development of diabetic complications is directly attributed to pro-inflammatory cytokines.17 Insulin signaling and sensitivity are adversely affected by these cytokines. Type 2 diabetes, poor glucose tolerance, and TNF-α, IL-6, and IL-1β are all associated with these conditions.17 The phosphorylation of insulin receptor substrate- (IRS-) and protein kinase B (PKB/AKT) has been shown to be suppressed by proinflammatory cytokines, which decreases insulin signaling.18 Our most recent findings shown that controlling the levels of adipocytokines led to a considerable decrease in TNF-α, IL-1β, and IL-6 as well as an important elevation of anti-inflammatory cytokine, IL-10, in diabetic rats.17 Adipocytokines' anti-inflammatory and antioxidant properties thus has essential role in the insulin and antihyperglycemic sensitizing effects of adipocytokines, at least in part.
Oxidative stress in metabolic disease
Reactive oxygen species are believed to cause metabolic diseases by promoting processes that result in the development of metabolic problems in cells that have been exposed to glucose.19 Increased metabolic illness oxidative stress may be brought on by the ongoing production of ROS brought on by metabolic diseases and glucose autoxidation.20 As a result, metabolic diseases can cause oxidative stress, which in turn can affect glucose, insulin signaling and fatty acid metabolism thereby leading to insulin resistance. Enhanced liver lipid peroxidation and decreased antioxidants, isocitrate dehydrogenase (ICDH), were indicators of oxidative stress using a model of disturbed-metabolic homeostasis in rats.17
In metabolic disorder, trigger and peroxidation of polyunsaturated fatty acids in cell membranes can be initiated by reactive oxygen species (ROS), which can cause cell damage. The oxidative stress that results from this is a significant contributor to the emergence of metabolic problems such nephropathy and retinopathy. It is possible that changes in antioxidant status may also affect how severe oxidant-mediated damage occurs, as metabolic illness can also cause decreased endogenous cellular antioxidant activity in a number of organs. The enzyme isocitrate dehydrogenase (ICDH) catalyzes the decarboxylation of isocitrate to –ketoglutarate, through creation of Nicotinamide adenine dinucleotide hydrogen acceptor (NADH) and Reduced nicotinamide adenine dinucleotide phosphate (NADPH) from NAD+ or NADP+. 21
According to Reitman et al.21 for the normal functioning of the NADPH, a crucial reducing equilibrant which is also relevant for the regeneration of reduced glutathione (GSH) prevents oxidative damage. Consequently, during oxidative stress, ICDH might function as an antioxidant. To prevent mitochondrial and cytosolic oxidative damage, ICDH is necessary for providing NADPH which helps in the synthesis of GSH22 The imbalance between oxidants and antioxidants may therefore be upset as a result of ICDH damage, which would then result in a pro-oxidant situation.
Transcriptional factors associated adipokines, known as adipogenic transcription factors are known lineage-determining factors which play an important role in the control of adipogenesis. Peroxisome proliferator-activated receptor gamma (PPARγ), Carbohydrate-responsive element-binding protein (ChREBP), sterol regulatory element binding protein-1c (SREBP-1c) and Orphan nuclear receptor 4A1 (NR4A1) are all master “adipogenic” transcription factors whose mechanistic pathway could be target for the management of metabolic disorder etc.
Molecular pathways of adipocyte differentiation, insulin sensitivity, and inflammation are all influenced by an important transcription factor, peroxisome proliferator-activated receptor (PPAR), which is crucial for the growth and function of adipose tissue. PPAR-γ is active during differentiation, and this stimulates genes production specific for adipose tissue that are involved in the phenotype of adipose tissue and glucose metabolism23. Additionally, pro-inflammatory transcription factors are inhibited by active PPAR-γ, which reduces the expression of pro-inflammatory cytokines and the inflammation-related state in adipose tissue24 Many known PPAR2 targets are expressed by adiponectin, G0/G1 switch 2 (G0s2), fatty acid-binding protein 4 (Fabp4), and other NR4A1-regulated genes (Adipoq). These data suggest that NR4A1 and PPAR2 have a biological function in common. The NR4A1 N-terminal domain is required for the regulation of the PPAR2 promoter since it is a direct target of NR4A1-dependent repressive regulation.18
Insulin, which is so released by the pancreas after a high carbohydrate meal, is one of the main primary activator of hepatic lipogenesis. Insulin activates SREBP-1c while glucose activates ChREBP independently of insulin.63,67 Notwithstanding, SREBP-1c and ChREBP are known transcriptional regulators of fatty acid (FA) and triglyceride (TG) production and are usually produced in response to insulin stimulation. SREBP-1c and ChREBP are classified as key lipogenic enzymes that promote lipogenesis in the liver. However, uncontrolled de novo lipogenesis (DNL) results in hepatic steatosis, which is usually linked to the development of obesity, insulin resistance, and type 2 diabetes. Hepatic steatosis is caused by excessive lipogenesis caused by transgenic overexpression of SREBP-1, a high fructose diet, or a lack of leptin.
SREBP-1c expression in the liver is decreased in intermittent fasting and exercised animals, which could be as a result in a rise in insulin levels.63,67 SREBP-1c expression was decreased in streptozotocin-induced diabetic rats, demonstrating that insulin mediates SREBP-1c induction63,67
Furthermore, liver-specific deletion of ChREBP has been shown to significantly improved fatty liver disease and overall glucose tolerance as well as insulin sensitivity in mice, potentially by lowering de novo lipogenesis69. Thus, overexpression of activated ChREBP in the liver is known to increase hepatic stestosis, which could be related to increased expression of genes in the liver that regulate fatty acid and TG production.
Inhibiting overexpression of SREBP-1 and ChREBP, on the other hand, could be a possible therapeutic method for treating dyslipidemia and metabolic syndrome.
Nuclear orphan receptor (NR4A) superfamily members include orphan nuclear receptor 4A1 (NR4A1), a transcriptional member that has recently attracted interest in a variety of scientific fields.25 The ligand-independent NR4A1 gene has been proven to be an immediate-early response gene, and various stimuli can easily promote the production of the protein.24 Numerous metabolic processes, including glucose metabolism, lipid metabolism, and energy balance are impacted by the hyperactivity or malfunction of NR4A1,25–27 This involves important metabolic organs such the liver, skeletal muscle, pancreatic cells, and adipose tissues. In response to inflammatory cytokines like TNF, IL-1, oxidized lipids, and lipopolysaccharide (LPS), NR4A1 is necessary for a number of cellular activities.28,29 The powerful anti-inflammatory actions of NR4A1 in inflammatory diseases have recently attracted attention.28 Genetic research identified NR4A1 as having a crucial role in the regulation of inflammation which is underscored through its protective role in atherosclerosis and obesity.30 Glucose uptake, and oxidation, skeletal muscle development, glycogen synthesis, and other glucose-metabolizing processes are all tightly correlated with the NR4A1 receptor13.32 Furthermore, lipid metabolism in muscle is regulated by the NR4A1 receptor. An essential metabolic function of NR4A1 is to control muscle lipolysis.32 When lipolysis is impaired and fat accumulates as a result of reduced NR4A1 expression, obesity develops. Furthermore, NR4A1 is essential for controlling the lipid metabolism in adipose tissue. Adipose tissue plays a key role in maintaining a healthy weight, and NR4A1 is related to the regulation of fat content.33 The fact that animals with NR4A1 deficiencies gain more weight and fat mass than mice of the same genotype when fed a high-fat diet supports the hypothesis that NR4A1 regulates lipolysis and energy expenditure. NR4A1 expression is significantly decreased in mice with diabetes and obese models.34
Additionally, NR4A1 expression has been shown to control a number of essential genes involved in lipid metabolism and lower the production of hepatic triglycerides in a study that was supported by data.35 The low density lipoprotein (LDL) receptor, fatty acid synthase (FAS), mitochondrial glycerol-3-phosphate acyltransferase (GPAT), SCD-1, and NR4A1 are some of the enzymes that are affected by the suppression of SREBP1c production.36 One way that NR4A1 alters hepatic lipid metabolism is through suppressing SREBP1c activity
Adiponectin is produced by adipose tissue and has a molecular weight of 30kDa.37 In a diabetic rat model, the adiponectin overexpression has been reported to possess anti-diabetic impact. Since adipokines is essential in immunity, anti-inflammatory, neuroendocrine function, energy homeostasis, insulin-sensitization, glucose regulation, lipid metabolism, and anti-atherogenic function, serum levels of ghrelin and adiponectin could be measured to determine systemic metabolic homeostasis.38-43 Adiponectin has been proven in studies to affect insulin sensitivity in a model of disturbed metabolic homeostasis in rats.44 Low levels of adiponectin levels are reported in people with obesity, type 2 diabetes and coronary artery disease.45 A connection between adipocytokines and metabolic disease reflects that diabetic rats exhibit decreased serum adiponectin concentration.46,47 Through inhibition of gluconeogenesis and enhanced lipid oxidation, adiponectin has additionally been shown to activate AMP-activated protein kinase (AMPK) thereby controlling glucose metabolism and insulin sensitivity.48
Moreover, adiponectin inhibits hepatic gluconeogenesis through its lowering effect of glucose-6-phosphatase and phosphoenolpyruvate carboxylase, which results in a decreased hepatic glucose production48-49. Importantly, adiponectin can reduce triglyceride content in the skeletal muscle and liver, activate the PPAR-γ (peroxisome proliferator activated receptor), and increase the oxidation/combustion of muscle/liver fat by inhibiting acetyl-CoA carboxylase.38,48 By means of these pathways, adiponectin and ghrelin help to increase insulin-induced signal transduction, which in turn increases insulin sensitivity.48 The cause of the reduced adiponectin levels in insulin resistance has not been clearly identified. One of the molecules responsible for development of insulin resistance is TNF-α. TNF-α significantly decreased insulin sensitivity and the amount of adiponectin produced by adipocytes in a rat’s model of impaired systemic metabolic homeostasis by deactivating its promoter activity PPAR-γ, and activating a vital transcription factor, sterol regulatory element binding protein-1c (SREBP-1c).67 Although a known insulin mediator which is involved in insulin sensitivity, adipogenesis and lipid metabolism.50
Moreover, it has been discovered that lower serum levels of ghrelin are linked to obesity, insulin resistance, impaired insulin sensitivity, and type 2 diabetes.51 Furthermore, through the via a GHSR-specific mechanism, ghrelin therapy has been demonstrated to reduce peripheral blood mononuclear cell (PBMC) production of proinflammatory cytokines (TNF-α, interleukin IL6 and IL1).52 Ghrelin was also demonstrated to decrease IL6 and TNF mRNA expression in primary human T cells, pointing to a possible role for ghrelin in the transcriptional regulation of inflammatory cytokine expression.53 So, it is possible to conclude that ghrelin is crucial in the control of diabetes due to its ability to inhibit proinflammatory cytokines.
Leptin acts primarily as a pro-inflammatory agent and by controlling cytokine synthesis and T cell activation, it makes people more susceptible to metabolic diseases.54 On the other hand, leptin stimulates PPAR-alpha to increase the oxidation of fatty acids in the liver.55 The severity of NAFLD was found to be more closely related to circulating leptin levels.56 Moreover, mutations in the leptin receptor (ObR) gene is associated with non-alcoholic fatty liver disease (NAFLD).56 Leptin therapy therefore appears to be extremely effective in hypoleptinemic lipodystrophic patients with non-alcoholic steatohepatitis (NASH) unlike NAFLD due to low levels of circulating adipokines57,58
A unique hormone called irisin offers a potential cure for the treatment of metabolic diseases. Irisin has been identified in a variety of tissues, including the cerebellum, cerebrospinal fluid, pineal gland, adult stomach, thyroid, pancreas, liver, spleen, testicles, and human fetuses. Novel adipomyokines like irisin are normally produced, activated, and transported to target sites to carry out their respective physiological activities. For instance, it can change white fat into brown fat with catabolic characteristics, boost the expression of uncoupling protein-1, coordinate the treatment of metabolic diseases like obesity and type 2 diabetes, increase energy consumption and glucose utilization and significantly lessen insulin resistance.59 Resistin, an adipokine that is regulated by PPAR, is thought to be responsible for adipose tissue malfunction. The physiological processes of inflammation,60 insulin resistance,60 eating behaviour,61 and energy metabolism have all been linked to resistin, according to reports.62 Resistin was found to be elevated in disturbed systemic metabolic balance in one of our earlier studies. This could be due to the deactivation of PPAR, an increase in SREBP, and a reduction in adiponectin brought on by diabetes.63 Nonetheless, research demonstrates that resistin reduces inflammatory activities in adipose tissue by modulating transcription factors and the adipokine signaling pathway, downregulating proinflammatory flux, and reducing inflammatory events.64 Treatment for polycystic ovarian syndrome, hypothyroidism, Prader-Willi syndrome, and other metabolic and endocrine diseases may involve using this drug's potential to affect adipose tissue and glycemic homeostasis. Hepatic resistin mRNA expression may also reduce during exercise and after fasting.65 It would be possible to support irisin's potential as a mediator of protection against diabetes, obesity and metabolic syndrome by demonstrating how it may affect the regulation of hepatic and pancreatic islet activities. As a result, it might be said that irisin mediated decreased resistin may be linked to improved glucose homeostasis, insulin signaling, and other aspects of the glycemic profile, thus, an attractive target in managing metabolic diseases.
Possible strategies for new therapeutic treatments for metabolic pathologies-related oxido-inflammatory diseases
PPAR-γ, ChREBP and SREBP-1c pathways, which are lipid-related pathways, may be the therapeutic strategy for treating metabolic diseases. It has been shown that thiazolidinediones (statins), ligands for this pathway and insulin sensitizers, as well as non-pharmacological therapy techniques like exercise and intermittent fasting, can control lipid metabolism and have anti-inflammatory effects. Exercise training increased the expression of NR4A1, PPAR-, and target genes, such as 2-AR, GLUT4, UCP3, and FAT/CD36, in skeletal muscle, despite the fact that these genes have been shown to be impaired in low capacity rats' skeletal muscles.63 Additionally, exercise training enhanced lipid and glucose metabolism. Fasting and adrenergic stimulation both cause NR4A1 to be expressed in white adipose tissue, while adrenergic stimulation also causes PPAR- to be expressed.63
Similar levels of ChREBP expression are found in the liver and pancreatic beta cells. ChREBP's physiological effects could promote beta cell proliferation as an adaptive reaction to rising insulin demand66. But because ChREBP is a crucial transcriptional regulator of lipogenesis in the liver and responds to glucose metabolites, an early theory suggested that ChREBP in beta cells might take part in glucolipotoxicity-mediated beta cell death. Hyperglycemia brought on by diabetes can lead to excessive islet de novo lipogenesis, which can harm beta cells. This toxicity may also impact other ChREBP targets. For instance, Txnip (thioredoxin interacting protein), which binds and blocks thioredoxin, a crucial antioxidant enzyme in beta cells. Beta cells are shielded from glucotoxic stress by txnip depletion. On the other hand, beta cell overexpression of Txnip results in apoptosis.66 As a result, ChREBP activation may significantly influence glucotoxicity through altering Txnip, suggesting that ChREBP activation is unsuitable for beta cells.
Diabetes and insulin resistance are characterized by hyperglycemia-induced TXNIP activated ChREBP overexpression, which results in pancreatic cell death, cardiomyopathy, metabolic problems, and a number of harmful effects. It stimulates NLRP3 (NLR Family Pyrin Domain Containing 3) and IL-1β, cytokines linked to insulin resistance and type 2 diabetes. Peroxisome proliferator-activated receptor alpha, glucose transporter-1 expression, endothelial nitric oxide production, and insulin synthesis are all impacted by TXNIP. TXNIP is overexpressed in malignancies, autoimmune disorders, nephropathy, diabetic retinopathy, and atherosclerosis, but it is not present in healthy cells.66
Interestingly, recent studies have focused on inhibiting beta cell Txnip to decrease glucotoxicity and treat diabetes. Notably, Verapamil has been identified as a drug that not only reduces TXNIP expression in pancreatic -cells, but also improves cell survival and function, potentially preventing diabetes. Also, calcium channel blockers (CCB) used to treat hypertension have been shown to suppress TXNIP expression in cardiomyocytes.66
More so, Adipocytokines including leptin, irisin, ghrelin, and adiponectin may be essential in the development of potential pharmaceutical treatments for oxido-inflammatory-related metabolic disorders. Exogenous adiponectin injection may offset the effects of metabolic pathology, such as leptin-induced inflammation, or it may stimulate the antiatherogenic, vasoprotective, and anticancer effects of the hormone. The suppression of leptin and resistin receptors is a potential option. In addition, as ICDH is implicated in NADPH production which is essential for GSH synthesis against cytosolic and mitochondrial oxidative damage, other potential targets may be reached by upregulating the ICDH antioxidant pathway.68 Our findings highlight the importance of identifying the molecules engaged in metabolic and oxido-inflammatory pathways, as well as molecules implicated in pathogen sensing systems, in order to understand the complex interactions between metabolic and oxido-inflammatory systems. By better defining and comprehending the mechanisms involved, this could pave the way for therapeutic strategies that would increase the capacity of endogenous molecules to ward off the inflammatory reactions and stress brought on by metabolic signals.
Prospects for future research
This review study demonstrates the Physiological modulation of adipogenic transcription factors target for success in resolving issues related to metabolic pathologies-mediated oxido-inflammatory responses brought on by excess adiposity. It is anticipated that performing additional experiments to address the anomalies of the mechanistic pathway of excess adiposity mediated metabolic pathologies using immunohistochemistry technique would be of clinical benefit in the management of metabolic disorder. Hence, in the future, various adipocytokines route that mediated control of oxido-inflammation via modulation of ICDH, PPAR-γ, NR4A1 and SREBP-1c will be very helpful in preventing metabolic disease.
A group of endocrine abnormalities known as metabolic disorders, which are becoming more and more common throughout the world, include obesity, diabetes mellitus, and insulin resistance. In conclusion, this review study shows that problems caused by poor metabolic homeostasis may be successfully resolved. Hence, in order to prevent the metabolic pathologies-related oxido-inflammatory responses various supplements acting via adipocytokines modulation of ICDH, PPAR-γ, NR4A1 and SREBP-1c signaling pathway will be useful in the future.
Limitation of the review study
The study's weakness is the dearth of information on the effects of particular drugs on metabolic process. Our goal was to review the various adipokines signaling pathways that could be targeted for the prevention of metabolic pathologies and to describe the change over time of metabolic function in a diagnostically diverse cohort of patients and animal experimentation relating to their level of adipogenic transcriptional activity. The purpose of this study was not to examine the influence of different medicines or pharmacological classes on the development of metabolic dysfunction, nor was it powered to do so.
None.
Author declares there are no confits of interest.
None.

©2023 Mega, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.