eISSN: 2373-6372


Background: The purpose of this study was to compare the primary patency of 7F vs 10F plastic biliary stents (PBS) placed by percutaneous transhepatic technique in two cohort of patients that received palliative treatment for malignant biliary obstruction.
Materials and methods: From January/2016 to April/2020, 34 patients who were admitted to a tertiary academic center because of malignant biliary obstruction were enrolled in a prospective non-randomized study. Between January/2016 to December/2018, 7F PBS implants were performed and between January/2019 to January/2021, 10F PBS implants were performed.
Results: The technical successful rates of PBS 7F and 10F were 100% (16/16) and 100% (18/18), respectively (P=1.000). The primary patency was 153.2days for 7F PBS and 230.3days for 10F prosthesis, with a statistically significant difference (P=0.014). The average survival time in the group of patients who used the 7F prosthesis was 230.0 days and that of the 10F prosthesis was 242.0days, with no statistically significant difference (P=0.840). For 7F and 10F groups, stent dysfunctions were found in 7 and 4 patients, respectively (43.7% vs 22%, P=0.434). There was no significant difference between the two groups in the incidence of serious adverse events.
Conclusions: 10F plastic stents had a longer duration of patency than 7F which recommends their use in the palliative treatment of patients with biliary obstruction due to malignant biliary obstruction.
Keywords: obstructive jaundice, biliary obstruction, percutaneous drainage, biliary stent, radiology, interventional radiology.
Keypoints: The 10F plastic stents had a longer duration of patency than 7F which recommends their use in the palliative treatment of patients with biliary obstruction due to malignant biliary obstruction.
PBS, plastic biliary stents, ERCP, endoscopic retrograde cholangiopancreatography; PTDB, percutaneous transhepatic biliary drainage, PBS, plastic biliary stents; SEMS, self-expanding metal stents, PTH, percutaneous transhepatic; SOJ, symptomatic obstructive jaundice; CT, computed tomography;
Endoscopic retrograde cholangiopancreatography (ERCP) is the usual approach for patients with distal malignant biliary obstruction.1 However, even when performed by experienced endoscopists, ERCP has a failure rate of 3-12%.2 Percutaneous transhepatic biliary drainage (PTBD) can be used for patients with distal and proximal malignant biliary obstruction or in cases where ERCP fails or cannot be performed, with excellent results.3,4 Regarding palliative treatment, a meta-analysis published in 2017 showed that, as compared to plastic biliary stents (PBS), self-expanding metal stents (SEMS) are associated with longer stent patency and duration of symptomatic improvement, less late complications and reinterventions, but without impact in overall survival.5 Due to SEMS cost 15 to 40times more than most PBS, SEMS seems to be less cost-effective in patients with life expectancy lower than four months.6
Recently, Nunes TF et al. described a novel technique of percutaneous transhepatic (PTH) placement of a 7 French (F) PBS with 100% of primary patency in 90 days, with a mean follow-up of 349.4 days, for palliative malignant biliary obstruction.7 In case of advanced unresectable hilar malignancies, palliation with PTBD seems to be superior to ERCP.1,8 Newly, Nunes TF et al. started to use a 10F instead of 7F PBS for PTBD of palliative malignant biliary obstruction and it seems better results were achieved.9
The purpose of this study was to compare the primary patency of 7F vs10F plastic biliary stents placed by percutaneous transhepatic technique in two cohort of patients that received palliative treatment for malignant biliary obstruction.
Study design
This was a retrospective, open-label, double-arm, single-center of a cohort of patients with malignant biliary obstruction undergoing PTH placement of a PBS to relieve obstruction. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (CAAE: 22858019.5.0000.0021) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants.
Patient selection
Eligible participants were all patients with symptomatic obstructive jaundice (SOJ) related to malignant biliary obstruction who underwent PBS from January 1, 2016 to April 30, 2020 for palliative treatment (Figure 1). Between January 1, 2016 and December 31, 2018, 7F PBS implants were performed and from January 1, 2019 until January 1 2021, the 10F PBS.
The patients were evaluated with ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) and the results of the exams were discussed in an Oncology Board to define tumor resectability or not. Although in the initial sample there were a significant number of patients with obstructive jaundice associated with metastatic disease, we chose to select only patients with primary unresectable disease, since the main objective of the study is to assess the primary patency between the two prostheses. Data were collected from the patients’ electronic medical records, diagnostic imaging tests, and laboratory tests. All PBS placement procedures were performed by a single interventional radiologist with 10 years of experience in biliary interventions procedures, assisted by two residents in interventional radiology. The inclusion and exclusion criteria are listed in Table 1.
Inclusion criteria |
Exclusion criteria |
Age > 18years; |
Potentially resectable tumor; |
Definite diagnosis of malignant biliary obstruction per cholangiobiopsy; |
Metastatic disease; |
Tumor irresistibility; |
Patients previously treated with a biliary ERCP; |
Signs of cholestasis (2 of 3): Ultrasound showing dilation of intrahepatic or extrahepatic; bile duct; Bilirubin ≥2mg/dL or increase ≥1mg/dL; or elevated AP/GGT more than twice the normal value or increase of at least 30U/L. |
Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥4;
|
|
Severely dysfunctional heart, kidney, lung, and coagulation; |
|
Inability to provide informed consent. |
Table 1 Inclusion and exclusion criteria
Level of obstruction
To determine and classify the level of obstruction we decided to use a modified Bismuth-Cortelle classification.10
Institutional protocol in the approach of obstructive jaundice
The protocol in our institution for patients with high obstructive jaundice (e.g., malignant lesions, Bismuth type II, III, or IV) is transhepatic PBS placement and cholangiobiopsy with reassessment by the endoscopy team after 3months. Low obstructive jaundice (e.g., malignant lesions, Bismuth type I) cases are referred for endoscopic biliary drainage and, if unsuccessful, they are referred to the interventional radiology unit. The same protocol is adopted for benign lesions. Three months after biliary drainage with a PBS, patients are referred to the endoscopy unit for assessment of the position of the previous transhepatically placed stent and possible removal in benign cases. If malignancy is confirmed, the stent should be replaced with a new plastic or metal stent based on the availability of materials. For patients with signs of cholangitis, which was an exclusion criterion in the present study, in addition to a PBS, an intrahepatic 8F external pigtail catheter was also placed until the infectious process resolved.
Technical description
Patients received cefazolin 1g intravenously, but patients with a history of biliary intervention received cefotetan 2g intravenously. Local anesthesia with 2% lidocaine (10mL) was administered at the percutaneous puncture site previously determined on imaging tests under conscious sedation. Ultrasound-guided PTH puncture of a peripheral bile duct was performed with a 17 Gauge (G) x 10.6cm coaxial needle (Argon Medical Devices, Inc.) and a 5F radial sheath (Radifocus Introducer II, Terumo®, Tokyo, Japan). Cholangiography using a common contrast medium (iohexol - Omnipaque™ 300, GE Healthcare, Milan, Italy, diluted 1:1 with saline) was then performed to visualize the location of obstruction.
Technical and clinical success and patency
Technical success was defined as adequate PBS deployment, with one post papillary end and the other pre tumor, with adequate drainage of the bile duct as determined by cholangiography after the procedure and abdominal radiographs.
Clinical success was based on the improvement of symptoms (pruritus, jaundice, nausea, vomiting, choluria, and fecal acholia) and defined as a reduction by at least 30% in the level of total bilirubin within 2weeks after stenting.
Primary patency was defined as the period from stent insertion to stent dysfunction, patient death, or the date of the last follow-up.
Definition of stent occlusion
Stent dysfunction was defined as a recurrence of jaundice and/or cholangitis due to tumor growth (ingrowth or overgrowth), sludge, stent migration, or other reasons.
Stent failure was assumed when two of the three criteria were fulfilled: 1 - Ultrasound showing new dilation of intra-hepatic or extrahepatic bile ducts; 2 - Bilirubin ≥2mg/dl with increase ≥1mg/dl compared with the value after initial successful drainage, or elevation of alkaline phosphatase and/or gamma glutamyl transferase more than twice the normal value with an increase of at least 30U/l; 3 -Signs of cholangitis (fever and leukocyte count >10×109/l or C-reactive protein > 20mg/dl). In case of stent dysfunction, the PBS was replaced by a SEMS.
Follow‐up
For both groups, post-stenting follow-up was routinely performed at 2weeks, 1 and 3months, and then every 3months, and included physical examination, CT scan, and liver function test. The follow-up time was 24months for both groups, although the periods for performing the procedure were at different times. The patients were advised to visit the hospital any time if they experienced recurrent cholangitis or any obstructive jaundice symptom.
At month 3, the PBS was assessed by the endoscopy team, the endoscopic approach stent aimed the replacement of the previous percutaneously placed stent with an endoscopically placed plastic or metal biliary stent. Endoscopic access was gained to the major duodenal papilla using an Olympus TJF-150 duodenoscope, with the patient in prone position and under deep sedation administered by an anesthesiologist. Stents were removed by grasping the distal end of the stent and withdrawing it using a polypectomy snare. In cases of stent replacement, selective cannulation of the bile duct was performed with a 0.035” hydrophilic guidewire (Hydra Jagwire, Boston Scientific®) guided by an Olympus CleverCut 3V sphincterotome, 50% iodinated contrast solution injection for cholangiography, papillotomy, and placement of a Boston Scientific Flexima 10Fr plastic stent or a WallFlex Biliary RX self-expanding metal stent.
Endpoints
The main endpoint was to compare the primary patency of percutaneous transhepatic placement of two different sizes of PBS, 7 and 10F, for the treatment of malignant biliary obstruction. The secondary endpoints included technical success, clinical success, adverse events, and survival.
Adverse events and complications
Complications were classified according to the 6-grade CIRSE classification system .11
Statistical analysis
Data were entered into the Excel program and later exported to the IBM SPSS statistics version 20.0 program for statistical analysis. The symmetry of the variable was assessed using the Kolmorogov Smirnov test. Quantitative variables with symmetric distribution were described by means and standard deviations and compared by Students test for independent samples. Categorical variables were described by frequencies and percentages and compared using the Chi-square test or Fishers exact test according to application conditions. Patency and survival time were analyzed using the Kaplan Meier analysis and the log rank test was used to compare survival curves. Cox regression was used to adjust the relationships between the prostheses and the patient`s survival for potential confounding factors (including those factors with a statistically significant difference between the groups). A significance level of 5% was considered, and differences were considered statistically significant when the P-value was ≤ 0.05.
Baseline
Data were collected for 34 patients, 14 females, with a mean age of approximately 65 years (range from 49 to 88 years, standard deviation 7years). Of the 34 patients, 16 (47.1%) used the 7F prosthesis and 18 (52,9%) used the 10F prosthesis. Considering the pre-procedure variables (baseline), when comparing the groups, there were no statistically significant differences for age, sex, level of obstruction (Bismuth), total bilirubin, site of drainage and anticancer therapy, demonstrating the homogeneity between the groups studied. Table 2 shows all the results.
|
Plastic Biliary Stent |
|
|
7 F |
10 F |
P |
|
Age (years), average±SD |
65.8±9.0 |
63.4±2.6 |
0.324 |
Sex male, n (%) |
8 (50.0) |
9 (50.0) |
1.000 |
Bismuth, n (%) |
|
|
0.501 |
I |
6 (37.5) |
4 (22.2) |
|
II |
1 (6.2) |
2 (11.1) |
|
III |
2 (12.5) |
6 (33.3) |
|
IV |
7 (43.8) |
6 (33.3) |
|
Biopsy results, n (%) |
|
|
0.999 |
Hilar malignant biliary obstruction |
11 (68.8) |
12 (66.7) |
|
Distal malignant biliary obstruction |
5 (31.2) |
6 (33.3) |
|
Total bilirubin before, average±SD |
15.20±3.94 |
13.99±1.53 |
0.276 |
Total bilirubin 30-60days after, average±SD |
1.24±0.26 |
1.32±0.33 |
0.128 |
Site of drainage, n (%) |
|
|
0.999 |
Unilateral |
16 (100) |
18 (100) |
|
Complications, n (%) |
2 (12.5) |
3 (16.6) |
0.999 |
Anticancer therapy, n (%) |
|
|
0.999 |
Chemotherapy |
14 (87.5) |
14 (77.7) |
|
Chemoradiation therapy |
2 (12.5) |
4 (22.2) |
|
Radiation |
- |
- |
|
PBS replaced by a SEMS, n (%) |
7 (43.8) |
4 (22.2) |
0.434 |
Table 2 Baseline, site of drainage and complications
F, french; SD, standard deviation; PBS, plastic biliary stent; SEMS, self-expanding metal stent
Categorical variables associated by the Chi-square test or Fisher’s exact test and quantitative by Studenst’s test for independent samples.
Technical success
The technical successful rates of PBS 7F and 10F were 100% (16/16) and 100% (18/18), respectively (P=1.000). The lengths of both stents were 12cm. All stents in the 7F and 10F group were inserted unilaterally using the right side (Table 2).
Stent patency
The primary patency was 153.2 days for 7F PBS (95% confidence interval: 130.5 to 175.9 days) (Figure 2) and 230.3days for 10F prosthesis (95% confidence interval: 214.0 to 246.7days) (Figure 3) with a statistically significant difference (P=0.014) (Figure 4).
Stent dysfunction
For groups 7F and 10F, stent dysfunctions were found in 7 and 4 patients, respectively (43.7% vs 22%, P=0.434). Among these 11 patients, nine had sludge and 2 had tumor growth. Tumor growth and sludge were treated with SEMS insertion transhepatically (N=7) and endoscopically (N=4).
In the PBS 7F group, the earliest dysfunction occurred 95days after stent implantation, whereas in the 10F group, the earliest stent dysfunction occurred 170days after implantation, therefore almost twice as long.
Survival
The median survival time in the group of patients who used the 7F prosthesis was 230.0 days (95% confidence interval: 176.7 to 283.0days) and the 10F prosthesis was 242.0days (95% confidence interval: 206.1 278.0days) with no statistically significant difference (P=0.840) (Figure 5). In the presence of a statistically significant difference between the two groups for total bilirubin 30-60days after PBS implantation, adjustments of patient survival comparisons for this factor were performed using Cox Regression. Comparing patients who used 7F PBS and 10F in regarding survival, there is still no significant difference after adjusting for total bilirubin after 30-60 days (P=0.236).
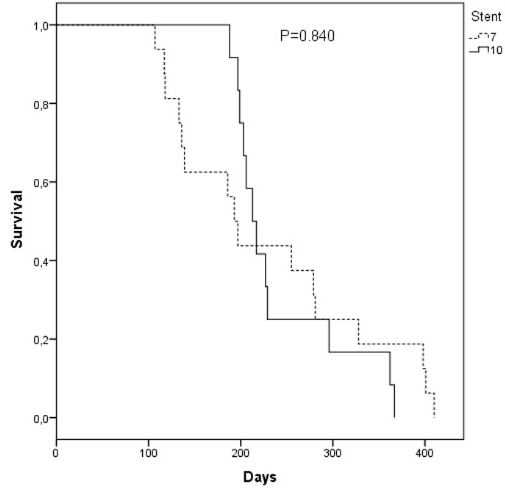
Figure 5 Comparative curve of the accumulated survival in days in the groups of patients with the 7F and 10F prostheses.
Adverse events and complications
In the group of patients who used 7F PBS, adverse events included cholangitis (n=1) and self-limited bleeding (n=1). In the group of patients using 10F PBS, adverse events included self-limited bleeding (n=1), perihepatic bilioma (n=1) and pancreatitis (n=1).
The introduction of the transhepathic percutaneous insertion technique of endoscopic plastic biliary prostheses has determined potential advantages in terms of feasibility, safety, and effectiveness.7 We believe that patients who will benefit most from this technique are those with unresectable malignancy who had failed endoscopic therapy (ERCP), those with hilar lesions, and cases of diagnostic uncertainty about the cause of obstruction.
Our results demonstrated technical and clinical success in 100% of patients in both groups (7F versus 10F plastic prostheses). The primary patency was significantly higher in the group that used 10F PBS with 230.3days (95% confidence interval: from 214.0 to 246.7days) versus 153.2days (95% confidence interval: 130.5 to 175.9days) of the 7F prosthesis (P=0.014). In the 7F PBS group, the earliest dysfunction occurred 95days after the stent implantation, whereas in the 10F group, the earliest stent dysfunction occurred 170days after implantation, therefore almost twice as long. Around 170days after implantation, practically when all 7F stents were already occluded, 10F stents started to obstruct/occlude. We also note that the need to replace PBS with self-expandable metal stents (SEMS) was greater in the group that used 7F, 43% vs 25%, due to the greater number of obstructions. Another indication of the superiority, in terms of longer patency of 10F PBS over the 7F.
There were 2 complications in both groups, they were all minor complications. Cholangitis and self-limited bleeding were treated conservatively with increased antibiotic therapy time in the first case and observation and clinical control in the second. In the case of bilioma, treatment was successfully performed by percutaneous drainage. One patient with pancreatitis was not submitted to additional treatment, but there was an extension in the hospital stay by 7days.
For those patients in whom the biliary drainage procedure is part of palliative treatment and which aims to keep the bile duct open while the patient is alive (life expectancy from 3 months to 1 year), the ideal scenario is for the patient to do just one procedure and keep the bile duct open until death. In this sense, the use of PBS 10F was more efficient than 7F.
Doctor N et al.12 was the first author to describe the PTBD insertion technique of PBS (prosthesis caliber ranging from 7 to 14 F) with its initial results of 54 cases presenting a technical success rate of 89% (48 / 54) and primary patency around 80% of biliary prostheses in 270days. In his cohort, he obtained 12 PBS occlusions (two early <30days and ten late >30days) and in 100% (2/2) of early occlusions and 80% (8/10) of late occlusions, we observed that the caliber of PBS were 7F. The complication rate was 24%, the most common complication being cholangitis and the median survival for patients with palliation was approximately 3months.
Regarding palliative treatment, a meta-analysis published in 20174 showed that, as compared to plastic biliary stents (PBS), self-expanding metal stents (SEMS) are associated with longer stent patency and duration of symptomatic improvement, less late complications and reinterventions, but without impact in overall survival.5 One important limitation of SEMS use with biliary obstruction is the high upfront cost associated with their use. Therefore, if we have a PBS that manages to have the same length of time as a SEMS, with similar numbers of interventions and complications, it will be more cost-effective.
Perhaps the access route may have an influence on the duration of prosthesis. Duan et al.4 raised the hypothesis in their meta-analysis that the endoscopic approach did not show significant advantages over the percutaneous approach. PTBD resulted in a better rate of therapeutic success and a lower incidence of complications, leakage of intraperitoneal bile, mortality in 30days, sepsis, and duodenal perforation, compared to ERCP. Regarding cholangitis and pancreatitis, PTBD has also proved to be a superior method. In clinical practice, it is recommended to choose the method based on the location of the obstruction, drainage objective (such as preoperative procedure or palliative treatment) and level of experience in biliary drainage.
This study has some limitations. First, the study design did not allow comparison between the percutaneous and endoscopic techniques of placing plastic biliary prosthesis in terms of patency and safety rates. Second, the small sample size between the two groups. We noticed that there was a heterogeneity in the treated neoplasia groups, malignant distal biliary obstruction that involves periampular lesions with different prognosis and hilar malignant biliary obstruction.
We report our experience with PBP as a therapeutic modality of palliation for patients with malignant biliary obstruction. We have achieved high rates of technical and clinical success with minimal complications, suggesting that transhepathic placement of PBP can be a safe and effective method for the treatment of this oncological condition, which is supported by recent studies that address this topic. We believe that more case series are needed to show the effectiveness and replication of this method, so that the paradigm of percutaneous treatment with insertion of PBP in patients with inoperable malignant biliary neoplasia can be changed. The next steps for our clinical research would be to evaluate whether the insertion of multiple PBP are more effective than insertion of a single prosthesis and, finally, a prospective and randomized study comparing endoscopic and percutaneous techniques in the insertion of plastic biliary prosthesis in patients with unresectable malignant biliary neoplasia.
10F plastic stents had a longer patency time than 7F, which recommends their use in palliative treatment of patients with biliary obstruction due to malignancy.
None.
Author declares that there are no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.