eISSN: 2373-6372


Review Article Volume 10 Issue 4
1Departemnt of Liver Unit, University of Auckland, New Zealand
10Department for the Fight against Sexually Transmitted Infections and Viral Hepatitis, Ministry of Public Health, Namibia
11General Manager, Mylan Pharmaceuticals Private Limited, USA
12Manager, Medical Affairs, Mylan Pharmaceuticals Private Limited, USA
2Professor of Medicine, University of Chicago Biological Sciences, New Zealand
3Associate Professor, Lagos State University College of Medicine, Nigeria
4Director Coordinator, National Hepatitis Program, Nigeria
5Department of Medicine, University of Zimbabwe College of Health Sciences, Zimbabwe
6Head of the department, University Hospital, yalgado Ouedraogo, Burkina Faso
7Department of Hepato-Gastroenterology Université Cocody, Côte d'Ivoire
8Faculty of medicine and biomedical sciences, Melen street Yaoundé, Cameroon
9Specialist Physician, Internal Medicine, Ministry of Health and Social Service, Namibia
Correspondence: Edward John Gane, Liver Unit, University of Auckland, Auckland, 1010, New Zealand, Tel 6421548371
Received: June 26, 2019 | Published: July 22, 2019
Citation: Gane EJ, Charlton MR, Onyekwere CA, et al. Optimizing decision-making for initiating the treatment of hepatitis b virus infection in resource-limited settings: African consensus guidelines. Gastroenterol Hepatol Open Access. 2019;10(4):202?211. DOI: 10.15406/ghoa.2019.10.00383
Optimization of hepatitis B virus (HBV) testing and linkage to treatment may help curtail the HBV disease burden in Africa. Therefore, a panel of 10 experts from Africa convened, reviewed the literature and developed 10 recommendations for optimizing the diagnosis and treatment initiation for HBV infection in Africa. In resource-constrained African settings, a single hepatitis B surface antigen assay may be considered as the primary test for HBV diagnosis. Pre-treatment assessments should include tests for complete blood count, liver/renal function, hepatitis B e-antigen (HBeAg), anti-HBe, HBV DNA, co-infection, and disease severity assessment. Non-invasive alternatives to liver biopsy include aspartate aminotransferase-to-platelet ratio index , fibrosis score-4, and transient elastography. Antiviral therapy should be initiated in HBV-infected, cirrhotic individuals with detectable HBV DNA, regardless of alanine transaminase levels or HBeAg status. This consensus document may be a useful guide to clinicians for optimizing the diagnosis and treatment initiation for HBV infection in Africa.
Keywords: hepatitis B virus, diagnosis, consensus, Africa
WHO, world health organization; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; TE, transient elastography; AST, aspartate aminotransferase; ART, anti-retroviral therapy; APASL, association for the study of the liver, HCV, hepatitis C virus; ALT, alanine transaminases; PT, prothrombin time; ULN, upper limit of normal; GGT, gamma-glutamyl transpeptidase
Epidemiology and burden of hepatitis b virus infection in Africa
According to the 2017 World Health Organization (WHO) global hepatitis report, the worldwide prevalence of hepatitis B virus (HBV) infection in 2015 in the general population was 3.5% (257million individuals). In this report, the WHO African region was reported to have the second highest prevalence, with about 60million individuals living with HBV infection (6.1%).1 In a recent modelling study, involving 120 countries conducted by the Polaris Observatory Collaborators, the modelled global prevalence of hepatitis B surface antigen (HBsAg) infection in the general population was found to be 3.9% in 2016, corresponding to about 291million individuals. In this study, the modelled prevalence of HBsAg infection in the Central, East, Southern, and West regions of sub-Saharan Africa in 2016 was found to be 10.1%, 5.1%, 8.5%, and 9.8%, respectively (corresponding to approximately 12.2, 22.2, 6.8, and 39.1million HBsAg-positive individuals, respectively).2 Recent epidemiological studies conducted in Central African Republic and Gabon (Central sub-Saharan Africa) have also revealed a high prevalence of HBV infection, especially in asymptomatic young adults, pregnant women, children and adolescents.3,4 Other recent systematic reviews and meta-analyses have indicated a high prevalence of HBV infection in Cameroon and Ghana (West sub-Saharan Africa) in the general population, blood donors, and pregnant women.5,6 One of the key reasons for the high prevalence of HBV infection in Africa is the very low neonatal vaccination rates. In Africa, all 3 doses of HBV vaccine have been found to be administered to only 80% infants and the birth dose of the vaccine (within 24hours after birth) to only 10% neonates in 2016. Within Africa, the 3-dose HBV vaccination was administered to 86%, 88%, 86%, and 66%; and the birth dose of the vaccine was administered to 0%, <1%, 3% and 18% of infants in the Central, East, Southern and West sub-Saharan African regions, respectively. As a result of the low neonatal vaccination rates, the prevalence of HBsAg infection in children aged 5years has been found to be the highest in the WHO African region in 2016 (3.4%, corresponding to over 1million HBsAg-positive children aged 5years.2 Adding to this public health concern in Africa is the increasing burden of HBV-HIV co-infections. In the WHO report, the sub-Saharan African region has been cited to have the highest number of HIV–HBV co-infected individuals (1.96million 71% of the global HIV-HBV co-infected population).1 Several regional studies have also reported a high prevalence of HIV–HBV co-infection.7,8
In addition to the high prevalence, chronic HBV infection is also associated with high morbidity in Africa. A multicenter, observational cohort study conducted in various African countries revealed HBV infection to be a leading cause of hepatocellular carcinoma (HCC) in the Cameroon, Ethiopia, Ghana, Ivory Coast, Nigeria, Sudan, Tanzania, and Uganda regions of Africa.9 In other regional cross-sectional studies and series papers, HIV–HBV coinfection has been cited to be an important risk factor for aggressive liver disease, acute liver failure, HCC at a younger age, and significant fibrosis/cirrhosis.10,11 A statistical model based on a systematic review of data from 50 countries and data from GLOBOCAN 2012 revealed that about 50% of liver cancer cases in sub-Saharan Africa are caused due to HBV infection.12
The mortality from HBV infection has also been found to be high, both globally and in Africa. In the 2017 WHO global hepatitis report, about 1.34million global deaths were attributed to viral hepatitis in 2015. Of these deaths, about 66% were noted to be the result of complications due to chronic HBV infection. The African region stands third with respect to mortality from viral hepatitis, with about 136,000 deaths recorded in 2015.1 Furthermore, augmenting this burden is the clinical observation that the mortality from HBV-associated HCC occurs at a younger age (median: 38.9years) in sub-Saharan Africa, thus resulting in moreyears of life lost and greater economic loss in this region.1
One of the core interventions that can help curtail the morbidity and mortality from HBV infection is the scale-up of HBV testing and timely linkage to treatment and care.1,11 However, there are several unmet needs in the diagnosis and initiation of treatment for HBV infection in Africa that hinder the effective implementation of this intervention.
Key unmet needs in the diagnosis and initiation of treatment of HBV infection in Africa
Hepatitis B virus infection remains largely underdiagnosed in Africa. A considerably low percentage of HBV-infected individuals in Africa are aware of their diagnosis.1 Identification of individuals infected with HBV and assessing the need to initiate treatment has been found to be difficult in Africa, due to the asymptomatic nature of chronic HBV infection and the economic and accessibility constraints associated with diagnostic testing.11 Although liver biopsy is the standard test for the assessment of fibrosis, its use in routine clinical settings in sub-Saharan Africa is limited due to its cost and invasive nature.11,13 The use of transient elastography (TE), a non-invasive method for diagnosing advanced fibrosis, is also limited in this region due to cost and accessibility issues and the need for trained operators.11,13,14 The aspartate aminotransferase (AST)-to-platelet ratio index (APRI) score is a WHO-preferred, cost-effective, non-invasive testing method that can help identify individuals with cirrhosis; however, its use in Africa has been reported to be minimal.11,13,14 Nucleic acid testing (NAT) or testing for HBV DNA, which is essential to detect individuals with high viremia, is costly and not easily accessible in Africa.11,14 Therefore, there is a clear unmet need for affordable, readily accessible, WHO-prequalified, point-of-care HBV testing services in low- and middle-income countries and resource-limited settings, such as Africa.11,14-17
Owing to the aforementioned barriers, HBV screening/testing rates have been found to be suboptimal in various regions in Africa.18,19 In a recent medical chart review of 3579 charts of patients initiating anti-retroviral therapy (ART), from large urban HIV treatment centers (n=12) across sub-Saharan Africa, systematic screening for HBV infection before initiating ART was found to be poor: less than a quarter of the patients were tested for the presence of HBV infection.18 Suboptimal screening has been also reported in other surveys conducted in low- and middle-income countries, including those from the African region.15 A clear need for targeted HBV screening of healthcare workers; medical waste handlers; commercial sex workers; people living with HIV; close contacts of HBV-infected individuals; hemodialysis patients; pregnant women; and individuals with established cirrhosis or decompensated liver disease has been highlighted in several regional studies.11,19
In addition to these screening and diagnostic gaps, it has also been noted that, due to easy access to ART, the HIV-HBV co-infected individuals in most African regions are administered life-saving ART regimens, most of which contain lamivudine, which has been found to cause the development of HBV-resistant mutants. This finding clearly highlights the unmet need for HBV screening prior to initiation of ART in this patient population.20,21
Other regional studies and surveys have highlighted the following additional major challenges to the scale-up of testing and timely linkage to treatment and care in Africa: (1) lack of national guidelines and strategic frameworks for HBV testing and screening; (2) lack of simplified diagnostic algorithms; and (3) the lack of clear referral pathways.11,14-16,19
In view of the high prevalence and burden of HBV, and the cited unmet needs in the diagnosis and initiation of treatment for HBV infection in Africa, a panel of 10 experts from 6 African countries convened to develop a consensus document to optimize decision-making and initiation of treatment of HBV infection in Africa. The expert panel reviewed the latest recommendations for the management of HBV infection from the Asian Pacific Association for the Study of the Liver (APASL), the European Association for the Study of the Liver (EASL), and the American Association for the Study of Liver Diseases (AASLD), and proposed recommendations for the diagnosis, pre-treatment assessment, and indications of therapy for HBV infection—along with recommendations for the management of special patient populations, such as those with cirrhosis, and co-infection in Africa. Algorithms were also developed to guide decision-making for the initiation of treatment in HBeAg-positive and HBeAg-negative treatment naïve patients.
This guidance document has been developed by the consensus of the expert panel based on: (1) formal review and analysis of relevant published literature, including various chronic HBV management guidelines; and (2) the panels’ experience in the management of HBV infection. The Grading of Recommendations Assessment, Development, and Evaluation system was not used for rating of the recommendations by the panel.
According to the APASL and AASLD guidelines, serological assays for HBsAg and hepatitis B surface antibodies (anti-HBs) should be conducted for the diagnosis of chronic HBV infection. Alternatively, testing for total hepatitis B core antibody (total anti-HBc) may also be done for the detection of HBV infection; however, additional HBsAg and anti-HBs testing may be required in anti-HBc-positive individuals to differentiate infection from immunity.22,23 Furthermore, the AASLD guidelines do not recommend the routine use of anti-HBc testing, and recommend it as a useful test primarily for the detection of HBV infection: (1) in patients who have HIV infection, or are about to initiate hepatitis C virus (HCV), anticancer or other immunosuppressive therapies or renal dialysis; and (2) in donated blood (or organs).23 Considering the high prevalence of HBV infection in African settings, the expert panel recommended the use of a single serological assay for the detection of HBsAg for the diagnosis of chronic HBV infection (Table 1).24
Recommendation number |
Recommendation |
I |
An assay for HBsAg is the primary test for the diagnosis of chronic HBV infection. |
Table 1 Recommendation for diagnosis of HBV infection
HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen
The initial assessment of all HBsAg-positive individuals should include: (1) detailed history-taking, including details of alcohol consumption, comorbidities, and risk factors; (2) evaluation of family history for cirrhosis or liver cancer; and (3) a thorough physical examination for identifying the presence of cirrhosis or decompensated liver disease. Other pre-treatment assessments should include the following (Table 2).
Recommendation number |
Recommendation |
II |
The initial evaluation of an individual with HBV infection should include detailed history-taking, family history assessment, thorough physical examination, assessment of complete blood count, and biochemical tests to assess liver/renal function. |
III |
Serological markers (HBeAg, anti-HBe, and HBV DNA) to assess the phase of infection and the extent of viral replication; and co-infection markers to rule out HAV, HCV, HDV, and HIV should be conducted as a part of the pre-treatment assessment. |
IV |
Non-invasive markers such as APRI, FIB-4, or TE may be used to assess the extent of fibrosis. The cut-off for APRI for the detection of significant fibrosis is ≥1.5, and that for cirrhosis is ≥2.0. A FIB-4 threshold >3.25 is indicative of advanced fibrosis and a threshold <1.45 is suggestive of minimal fibrosis. A TE measurement >9 kPa, and >12 kPa is suggestive of significant fibrosis and cirrhosis, respectively. In Africa, TE may a reasonable alternative to liver biopsy for the assessment of fibrosis in HBV-infected patients. Liver biopsy may be reserved for patients with a high viral load and high-normal or minimally raised ALT levels without clinical evidence of cirrhosis. If TE is not available or accessible, APRI or FIB-4 may be used as effective alternatives for the detection of liver fibrosis and cirrhosis. |
Table 2 Recommendations for pre-treatment assessments in individuals with chronic HBV infection
HBV, hepatitis B virus; HBeAg, hepatitis B e-antigen; anti-HBe, antibodies to HBeAg; DNA, deoxyribonucleic acid; HAV, hepatitis A virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; APRI, aspartate aminotransferase (AST)-to-platelet ratio index; FIB, fibrosis score; TE, transient elastography; ALT, alanine aminotransferase.
Complete blood count22-25
Liver function tests: Assessment of liver function should include tests for aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), bilirubin, albumin, and prothrombin time (PT).22-25 The upper limit of normal (ULN) for ALT for guiding the treatment of chronic HBV infection in African patients may be considered as 35 U/L in males, and 25U/L in females, in line with American guideline recommendations.23 Derangement of liver enzymes has been noted in both HBV-infected and HBV-HIV co-infected individuals in studies conducted in various African regions;26,27 elevated levels of aminotransferases have been found to significantly correlate with viral load in HBV-infected individuals. However, the use of liver enzyme levels as a single biomarker for assessing liver damage and/or disease progression is not encouraged. Assessment of liver enzymes should be done alongside other virological markers to assess the progression of HBV infection.26
Renal function test: Includes test for serum creatinine22
Virological tests: The virological markers that should be tested include HBeAg, antibodies to HBeAg (anti-HBe), and HBV DNA.22-25 The virological markers, HBeAg and anti-HBe have been found to be suggestive of active viral replication in HBV-infected individuals in regional studies. In these studies, the prevalence of HBeAg was found to be higher among HBV-infected individuals aged 0–20years, with a decrease in prevalence with increasing age; on the contrary, an increase in anti-HBe was noted with advancing age.28,29 Furthermore, HBeAg-positivity was found to be associated with a significantly higher risk for cirrhosis (11.2-fold) and HCC (6.7-fold), after adjusting for age, gender, and socioeconomic status in a prospective case-control study conducted in West Africa (asymptomatic HBV carriers, n=60; cirrhotic patients, n=53; and HCC cases, n=129).30 In the same study, a strong and significant correlation was noted between HBV DNA levels and cirrhosis and HCC, independent of the HBeAg status; high-level viremia (>2000IU/mL) was associated with a 17.3-fold and 38.8-fold increased risk of cirrhosis and HCC, respectively.30 Therefore, HBV DNA may be an important marker of viral replication, and may help guide decision-making regarding the initiation and monitoring of antiviral treatment in HBV-infected individuals.22,23,25
Assessment for markers of co-infection: Tests should be conducted for the detection of antibodies to hepatitis A, D, and C virus (anti-HAV, anti-HDV, and anti-HCV, respectively), and HIV, to exclude co-infection, or initiate appropriate treatment.22,25 Regional variations in the endemicity of HDV virus has been reported in recent studies, and hence, may be considered during assessment for makers of co-infection. In a systematic review and meta-analysis of 30 studies to assess the prevalence of HDV among HBsAg-positive individuals in sub-Saharan Africa, the pooled seroprevalence of HDV in Central Africa was noted to be 25.64% in the general population and 37.77% in individuals with liver disease. The corresponding rates in West Africa were noted to be 7.33% and 9.57%, respectively. In East and Southern Africa, the seroprevalence of HDV in the general population was noted to be much low at 0.05%. However, overall, a significantly higher chance of detection of anti-HDV was noted among HBsAg-positive patients with liver fibrosis or HCC compared to asymptomatic controls.31 A high prevalence of HBV and HDV co-infection in the Central African Republic has also been cited in other regional studies.3 Therefore, regional variations and the presence of concomitant liver disease may be considered while assessing for anti-HDV in HBV-infected individuals.
Histological evaluation of the extent of fibrosis and liver disease should also be a part of the pre-treatment assessment in HBV-infected individuals. Liver biopsy is considered as the reference standard for assessing the extent of fibrosis. However, its use may be subject to sampling errors, interpretational variability, and safety concerns owing to its invasive nature.22,32 Therefore, a liver biopsy may be reserved for patients with a high viral load and high-normal or minimally raised ALT levels without clinical evidence of cirrhosis.22,25 An Ishak fibrosis stage ≥3 or METAVIR fibrosis score F ≥2 is indicative of significant fibrosis on liver biopsy.22,33 Furthermore, cirrhosis on liver biopsy refers to an Ishak fibrosis stages 5 and 6 or METAVIR fibrosis score of F4.22,33,34
The expert panel recommended the use of non-invasive tests such as APRI, fibrosis score 4 (FIB-4), or TE for the accurate assessment of the extent of hepatic fibrosis to guide treatment selection in HBV-infected individuals.22,24,25 The APRI is a simple and inexpensive test that can be easily performed in an outpatient setting. The cut-off for APRI for the detection of significant fibrosis is ≥1.5, and that for cirrhosis is ≥2.0.22 These thresholds have been successfully used in regional studies to assess the extent of fibrosis and cirrhosis. In a study conducted in Tanzania, HIV-HBV co-infected individuals (n=227) were found to have a significantly higher prevalence of APRI scores, suggestive of fibrosis and cirrhosis, than HIV-monoinfected individuals (n=2870). Furthermore, regression of APRI was noted in HIV-HBV co-infected individuals treated with appropriate ART.35 In another study conducted in Zambia that used the aforementioned APRI thresholds for the assessment of liver fibrosis and cirrhosis, it was noted that HBsAg-positive individuals were more likely to have significant liver fibrosis than HBsAg-negative individuals.36 The FIB-4 score is another non-invasive index that has been used to assess the extent of fibrosis in HBV-infected individuals. Cut-off FIB-4 scores of 1.45 and 3.25 have been found to identify the extent of HBV-related fibrosis.37 The expert panel recommended an FIB-4 threshold >3.25 to be indicative of advanced fibrosis and a threshold <1.45 to be suggestive of minimal fibrosis. These thresholds have been successfully used in two separate studies conducted in South Africa and North-Western Tanzania.38,39
The use of APRI and FIB-4 may not be ideal for the assessment of HBV-related fibrosis in patients with HBV infection with persistently normal ALT levels.40 Liver stiffness measurement using TE or Fibroscan® may be more useful in these patients.22,40 The expert panel recommended a liver stiffness measurement >9 kPa, and >12 kPa to be suggestive of significant fibrosis and cirrhosis, respectively in HBV-infected individuals.25 Closer thresholds have been used in studies conducted in various regions in Africa for the evaluation of fibrosis in HBV-infected and HIV-HBV co-infected individuals.41-44 In low-income settings such as Africa, TE may a reasonable alternative to liver biopsy for the assessment of fibrosis in HBV-infected patients.41 Furthermore, if TE is not available or accessible, APRI or FIB-4 may also be used as effective alternatives for the detection of liver fibrosis and cirrhosis, in resource-limited settings such as Africa (Table 2).24,25
In Africa, HBV infection is one of the leading causes of HCC.9,12,45 Contrary to the observation that HCC increases progressively with advancing age, the mean age of patients with HCC in Africa is considerably younger. Furthermore, the risk of HCC development, even in the absence of cirrhosis is high in HBV-infected individuals in endemic settings such as Africa.46 According to the latest European guidelines for the management of HCC, HCC surveillance should be offered to: (1) cirrhotic patients, Child-Pugh stage A and B; (2) cirrhotic patients, Child-Pugh stage C awaiting liver transplantation; (3) non-cirrhotic HBV-infected patients at intermediate or high HCC risk; and (4) non-cirrhotic F3 patients, regardless of etiology, based on individual risk assessment.46 However, it may be difficult to implement HCC surveillance in all the stated risk groups in Africa due to cost constraints. Furthermore, this scenario may be even more complicated by poor access to curative therapies such as resection in individuals detected with HCC.
In addition to these pre-treatment assessments, the AASLD guidelines recommend HBV genotype testing in selected patients, as some specific genotypes have been found to be associated with an increased risk of liver disease progression, and response to pegylated interferon alpha (PegIFN-α) therapy.23 An association between the HBV genotype and the risk of HCC has also been reported in the African region. Of the various HBV genotypes, type A1 is more prevalent in sub-Saharan Africa, type A3 is prevalent in West Africa, type D is prevalent in North Africa, and type E is prevalent in West and Central Africa. Of these genotypes, A1 has been found to be associated with a high rate of HCC, especially in young males, often in those without cirrhosis.47,48 Therefore, genotype testing may be useful in selected group of HBV-infected individuals. Furthermore, the EASL guidelines recommend HBsAg quantification in HBeAg-negative HBV-infected patients and in patients in whom IFN-α treatment is planned.22,25 However, the expert panel did not recommend these additional tests, considering the resource-limited settings in Africa.
The indications for initiation of antiviral therapy in individuals with chronic HBV infection should be based on a combination of three criteria: (1) serum HBV DNA levels; (2) serum ALT levels; and (3) severity of liver disease (assessed by clinical evaluation, non-invasive markers or liver biopsy). Indication for treatment should also consider other criteria such as age, family history of HCC or cirrhosis, and extrahepatic manifestations.22,25 The thresholds or the upper limit of normal (ULN) for ALT may be considered as 35U/L for males and 25U/L for females.23
The expert panel systematically compared the APASL, AASLD, and EASL guidelines and analyzed the recommendations from these guidelines for the initiation of therapy in compensated and decompensated cirrhotic individuals, and in non-cirrhotic HBeAg-positive and negative individuals, based on the above criteria.22,23,25 After reviewing the three guideline recommendations, the expert panel recommended that all HBV-infected, cirrhotic (compensated or decompensated) individuals with detectable serum HBV DNA levels should be started on antiviral therapy, regardless of the ALT levels or HBeAg status (Table 3). Initiation of antiviral therapy has been found to effectively control viral replication and improve the biochemical and clinical parameters in patients with compensated or decompensated HBV cirrhosis.49-51 Continuous treatment with antiviral therapy in HBV patients with advanced fibrosis or confirmed cirrhosis has been noted to prevent the clinical progression of the disease by significantly reducing the incidence of hepatic decompensation and the risk of HCC.52 Furthermore, regression of fibrosis and cirrhosis have been reported with long-term suppression of viral replication with antiviral therapy in HBV patients with advanced fibrosis.53 Considering the high morbidity and mortality from HBV infection, an aggressive approach involving the early detection and initiation of antiviral therapy may help reduce the incidence and mortality from HBV-associated HCC in resource-limited settings such as Africa.54
Recommendation number |
Recommendation |
V |
All HBV-infected, cirrhotic (compensated or decompensated) individuals with detectable serum HBV DNA levels should be started on antiviral therapy, irrespective of ALT levels or HBeAg status. |
Table 3 Recommendation for indications for therapy initiation in HBV-infected, cirrhotic individuals
HBV: Hepatitis B virus; HBeAg: Hepatitis B e-antigen; DNA: Deoxyribonucleic acid; ALT: Alanine aminotransferase.
The expert panel also developed two simple algorithms for easy guidance on the initiation of antiviral therapy in non-cirrhotic HBeAg-positive and -negative treatment-naïve individuals (Figures 1&2). The usage of these algorithms has been described in detail under recommendations 6 and 7 (Tables 4&5).
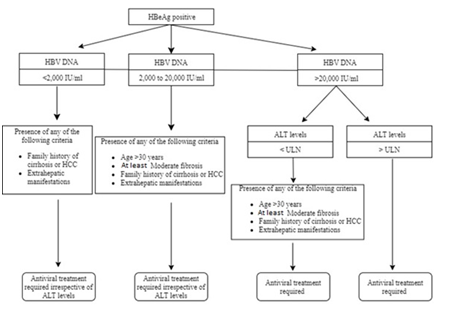
Figure 1 Algorithm to guide initiation of antiviral therapy in non-cirrhotic, HBeAg-positive, treatment-naïve, HBV-infected individuals.
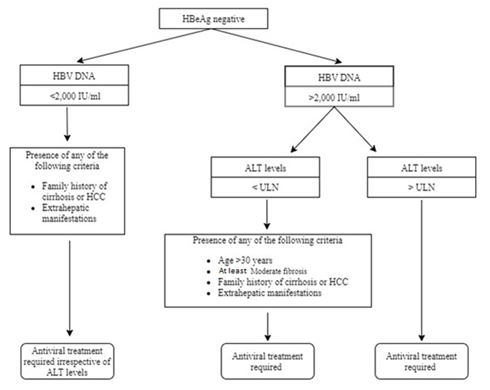
Figure 2 Algorithm to guide initiation of antiviral therapy in non-cirrhotic, HBeAg-negative, treatment-naïve, HBV-infected individuals.
Recommendation number |
Recommendation |
VI |
|
Table 4 Recommendation for guidance on therapy initiation in non-cirrhotic, HBeAg-positive, treatment-naïve, HBV-infected individuals (Figure 1)
HBV, hepatitis B virus; HBeAg, hepatitis B e-antigen; DNA, deoxyribonucleic acid; ALT, alanine aminotransferase; ULN, upper limit of normal; HCC, hepatocellular carcinoma
Recommendation number |
Recommendation |
VII |
|
Table 5 Recommendation for guidance on therapy initiation in non-cirrhotic, HBeAg-negative, treatment-naïve, HBV-infected individuals (Figure 2)
HBV, hepatitis b virus; HBeAg, hepatitis B e-antigen; DNA, deoxyribonucleic acid; ALT, alanine aminotransferase; ULN, upper limit of normal; HCC, hepatocellular carcinoma
Periodic assessment of serum ALT, HBV DNA levels and severity of liver fibrosis by non-invasive tests, should be conducted in all HBV-infected patients, not suitable for initiation of antiviral therapy: (1) every 3–6months in HBeAg-positive HBV-infected patients <30years of age; (2) every 6–12 months in HbeAg-negative HBV-infected patients with HBV DNA <2000IU/mL; and (3) every 3 months for the first oneyear and every 6months thereafter in HbeAg-negative HBV-infected patients with HBV DNA ≥2000IU/mL.25
Antiviral therapy may not be able to achieve complete HBV eradication or HBsAg loss in HBV-infected patients.51,55 Therefore, long-term antiviral treatment should be given in most chronic HBV-infected patients. Stopping of antiviral therapy may be considered after HBsAg loss, with or without seroconversion to anti-HBs. In HBV-infected, HbeAg-positive, non-cirrhotic patients, who have completed at least 12months of consolidation therapy, discontinuation of antiviral therapy may be considered if stable HBeAg seroconversion and undetectable HBV DNA have been noted. In HBV-infected, HBeAg-negative, non-cirrhotic patients, who have achieved ≥3years of virologic suppression, stopping of antiviral therapy may be considered.25 Literature suggests that on-treatment virologic suppression for >24months may be associated with greater chances of virologic remission after discontinuation of treatment in HBV-infected, HBeAg-negative patients.56
HIV Co-infection
All HIV-positive patients with HBV co-infection should be administered ART, irrespective of the CD4 cell count; tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF)-containing regimens should be used (Table 6). Both TDF- and TAF- containing regimens have been reported to be effective in causing virological suppression to undetectable levels in separate clinical studies and meta-analyses.57-59
HCV Co-Infection
Co-infection with HBV and HCV accelerates the progression of liver disease and increases the risk of HCC.60,61 All HCV RNA-positive patients with HBV co-infection should be considered for HCV direct-acting antiviral (DAA) therapy (Table 6). Treatment of HCV infection with DAAs may cause reactivation of HBV.62.63 Therefore, all HBV-HCV co-infected patients who fulfill the standard treatment criteria for HBV listed in the proposed algorithms (Figures 1&2) should receive HBV antiviral treatment. Other patients may be monitored by regular assessment of HBV DNA and ALT during HCV DAA therapy. Antiviral therapy for HBV infection may be initiated in these patients, if they develop HBV reactivation (defined as an increase in HBV DNA by at least 1 log plus elevation in ALT >ULN). Due to the resource-limited settings in Africa, if HBV DNA monitoring is not possible during HCV anti-viral therapy, anti-HBV therapy may be initiated in patients with a risk of HBV reactivation.
Recommendation number |
Recommendation |
VIII |
All HIV-positive patients with HBV co-infection should be administered ART, irrespective of the CD4 cell count. |
IX |
All HCV RNA-positive patients with HBV co-infection should be considered for HCV DAA therapy. All HBV-HCV co-infected patients who fulfil the standard treatment criteria for HBV listed in the proposed algorithms (Figures 1-2) should receive HBV antiviral treatment. |
X |
Pegylated interferon alpha for at least 48 weeks is the current treatment of choice for HDV-HBV co-infected patients. |
Table 6 Recommendations for indications for therapy in HBV-infected patients with co-infections
HBV, hepatitis B virus; HIV, human immunodeficiency virus; ART, anti-retroviral therapy; HCV, hepatitis C virus; DAA, direct-acting antivirals; HDV, hepatitis d virus
HDV Co-infection
Pegylated interferon alpha for at least 48weeks is the current treatment of choice for HDV-HBV co-infected patients (Table 6). Treatment with PegIFN-α has been found to be associated with an improvement in biochemical and virological parameters in chronic HDV infection.64
In HBV-HDV co-infected patients with active HBV replication, appropriate antiviral therapy should be initiated for the treatment of HBV infection.
The high prevalence of, and increased morbidity and mortality from, HBV infection in Africa necessitates an urgent need for the scale-up of diagnosis of HBV infection and timely linkage to treatment and care. Development of national HBV diagnostic guidelines and simple diagnostic algorithms will help provide guidance on the screening and diagnosis and parameters to be considered for decision-making on the initiation of treatment for HBV infection in Africa. The current African consensus guidelines provide comprehensive recommendations on the diagnosis, pre-treatment assessments, and indications for initiation of therapy in HBV-infected individuals, including for those with co-infection. Implementation of these recommendations in real-world clinical practice will help to a large extent in the scaling up of diagnosis and increasing the rates of treatment for HBV infection in Africa.
We would like to thank Mylan Pharmaceuticals ltd for financial support in execution of this project. We would also like to thank BioQuest Solutions Ltd for providing writing assistance.
Author Edward John Gane: Member of Clinical Advisory Board for Gilead Sciences, AbbVie, Janssen, Arrowhead, Merck, VIR Biotechnology, and Assembly Bio. Member of Speakers’ Bureau for Gilead Sciences, AbbVie, Mylan Pharmaceuticals
Author Michael Charlton: Consulting and research support for Gilead Sciences, Merck, AbbVie, Novartis and Only consulting support for Mylan
Author Nadia Jacqueline Mandeng: Transport and accommodation in the consensus building meetings was provided by Mylan
Author Ravishankar AC: Employee of Mylan Pharmaceuticals Private Limited
Author Sanjay Hadigal: Employee of Mylan Pharmaceuticals Private Limited
The project is supported by Mylan Pharmaceuticals Ltd.

©2019 Gane, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.