eISSN: 2373-6372


Research Article Volume 13 Issue 4
1Department of Biosciences, Federal University of Health Sciences of Porto Alegre (UFCSPA), Brazil
2Department of Medicine, Hepatology, Federal University of Health Sciences of Porto Alegre (UFCSPA), Brazil
Correspondence: Marilene Porawski, Department of Biosciences, Federal University of Health Sciences of Porto Alegre (UFCSPA), Brazil, Tel 5551 33038751
Received: July 27, 2022 | Published: August 3, 2022
Citation: Hahn GF, Nunes ML, Steffens L, et al. Omega-3 supplementation reduces oxidative stress and inflammation in the gut of obese rats. Gastroenterol Hepatol Open Access. 2022;13(4):138-143. DOI: 10.15406/ghoa.2022.13.00513
Obesity is a complex metabolic disease that does not have an effective treatment. It has been demonstrated that omega-3 fatty acids are beneficial to metabolism, but little is known about their effects in the gut. This study evaluated the effects of omega-3 supplementation in metabolic and intestinal changes caused by obesity. Obesity was induced by a high fat diet (HFD) for 20weeks in 40 rats. At 16th week, the animals were divided into 4 groups: standard diet (SD); SD+omega-3; HFD; and HFD+omega-3. Omega-3 groups were supplemented with omega-3 (1g/Kg) daily, for 4weeks. Food intake, biochemical parameters in the plasma, and markers of oxidative stress and inflammation in the gut were evaluated. HFD+omega-3 group had a decrease in total cholesterol, triglycerides and LDL compared to the HFD group. In the gut, omega-3 supplementation reduced reactive oxygen species production, lipoperoxidation, superoxide dismutase and catalase activity, NFκB activation and TNFα production. These results demonstrate that omega-3 supplementation plays a beneficial role in metabolic parameters and is able to decrease oxidative stress and intestinal inflammation in obese rats. Thus, omega-3 supplementation could be a useful tool in the treatment of obesity.
Keywords: obesity, polyunsaturated fatty acids, oxidative stress, NFκB, TNFα
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; TLRs, toll-like receptors; LPS, lipopolysaccharide; ROS, reactive oxygen species; HFD, high fat diet; SD, standard diet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase; SOD, superoxide dismutase; TNFα, Tumor necrosis factor alpha, NFκB, nuclear factor kappa B
Obesity is a multifactorial and polygenic disease,1 characterized by an excessive accumulation of fat in the adipose tissue and ectopic deposit of fat in non-adipose tissues.2 It is usually associated with dyslipidemia, hyperglycemia, insulin resistance, hyperleptinemia, mitochondrial dysfunction, endoplasmic reticulum stress and oxidative stress.2–4 All these changes promote a chronic and low-grade systemic inflammation that leads to a condition named metainflammation that produce a vicious cycle of unrestricted secretion of cytokines.5 Obesity is an important health issue worldwide due to the increasing consumption of highly processed, palatable and hypercaloric foods, typical of Western-style diet.6 In these hypercaloric diets, both saturated (SFA) and polyunsaturated (PUFA) fatty acids are a main source of energy. Despite SFAs are related to metabolic diseases, PUFAs from omega-3 family are beneficial to health.7 Omega-3 (n-3) and Omega-6 (n-6) are essential PUFAs. The beneficial effects of these fatty acids are well described when the ratio of n-6:n-3 decreases (6:1).8 During the last century there was an increase in the n-6 consumption and the ratio of n-6:n-3 increased from 5:1 to 16:1.9 This fact combined with the lower consumption of fish, a rich source of omega-3, contributes to the changes in the human metabolic pattern in the Western societies.10 The American Heart Association recommends consuming approximately 0.5g/day of eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) for cardiovascular disease risk reduction. However, in the years 2003–2008, more than 90% of people in the US consumed less than the recommended (median = 0.11g/day; mean = 0.17g/day).11 In this sense, omega-3 supplementation has been studied as an alternative to decrease metabolic dysfunction related to obesity. These studies presented encouraging results, such as: regulation of dyslipidemia, reduction of inflammation, reduction in the risk of cardiovascular disease, regulation of body weight, and prevention/ reversal of insulin resistance.12,13 However, little is known about the effects of omega-3 in the gut.
Gut plays an important role in diet-induced obesity.14 Obesity changes the composition of intestinal microbiota (dysbiosis), which is associated to an increase in the permeability of the intestinal epithelium.15 This allows the intestinal bacteria and their products, such as lipopolysaccharide (LPS) and toxins, to translocate and activate toll-like receptors (TLRs), promoting a cascade of proinflammatory cell signaling with the overproduction of inflammatory mediators and reactive oxygen species (ROS). Therefore, some alternatives have been studied to prevent, ameliorate or reverse the intestinal damage. Li and colleagues verified that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) prevent the disruption of the intestinal epithelial barrier, induced by proinflammatory cytokines in vitro.16 However; it is unknown whether omega-3 would diminish the proinflammatory profile in the intestine following obesity.
Therefore, the main objective of this study was to evaluate the metabolic effects of omega-3 supplementation in an experimental model of obesity induced by high fat diet (HFD) and its effect on inflammatory and oxidative stress parameters in the gut.
Animals
Forty adult Wistar male rats, weighing 150-200 grams, from the animal facility of the Federal University of Health Sciences of Porto Alegre (UFCSPA) were used in this study. The animals were kept in plastic boxes, with 2 animals per box, in an average temperature of 22 - 24°C, and light and dark cycle of 12 hours, having free access to food and water. All procedures were conducted in accordance with the provisions of the Arouca Law (Federal Law 11794/2008). The procedures in this study were approved by the Institutional Animal Care and Use Committee of UFCSPA, under protocol number 232/13.
Experimental design
Rats were randomly allocated into four groups (n =8-12/group): standard diet (SD); SD + omega-3; high fat diet (HFD); and HFD + omega-3. Diets were administered for 20weeks. The energetic content of standard diet (NUVITAL®, Curitiba, PR, Brazil) was 14.22kJ/g with 63% carbohydrates, 11% lipids, and 26% proteins. HFD had an energetic content of 18.83kJ/g (Pragsoluções, Jaú, SP, Brazil) with 35.7% carbohydrates, 45.1% lipids, and 19.2% proteins. After 16 weeks of diet, SD and HFD groups received 1mL of saline solution daily, and animals in SD+omega-3 and HFD+omega-3 groups were supplemented with omega-3 (1g/Kg) via gavage for 4 weeks. Feed intake was monitored daily.
Material collection
Animals were euthanized by overdose of the anesthetics xilasin hydrochloride (Rompum®) and ketamine hydrochloride (Ketalar®). Blood was collected through the retro-orbital plexus and placed in heparin tubes (Liquemine®). The blood was centrifuged at 2000g for 15minutes at 4°C, and the plasma stored in a freezer at -20°C. After ventral laparotomy, approximately 10 cm final portion of the gut were immediately removed, washed in PBS, frozen in liquid nitrogen, and stored at -80°C.
Analysis of biochemical parameters in plasma
Triglycerides, total cholesterol, HDL cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyltransferase (GGT), alkaline phosphatase, urea, and creatinine concentrations in the plasma were measured using an automatic biochemical analyzer (Vitalab Flexor E, Elitech Group). Analyses were performed using the commercial kits from Elitech Group according to the manufacturer’s instructions. LDL cholesterol was estimated by Friedewald formula: [LDL = (total cholesterol - HDL) - triglycerides/5].17
Tissue preparation and protein determination
Tissue homogenization was performed using a potter type homogenizer. The fresh tissue was weighed and homogenized with 1.5% KCl, containing a protease and phosphatase inhibitor cocktail (Sigma). Homogenates were centrifuged at 3000rpm for 10min at 4ºC, the pellet was discarded, and supernatants were kept at -80ºC until analysis. Protein concentration was determined by the method of Lowry using a standard curve of bovine serum albumin.
Analysis of oxidative stress in the gut
Lipoperoxidation induced by oxidative stress was measured by the technique of thiobarbituric acid-reactive substances (TBARS).18 One of the substances generated is malondialdehyde, when it is heated to 100°C, in the presence of thiobarbituric acid, a pink product is formed and measured by the spectrophotometer at 532nm. Results are expressed in nanomoles per milligram of protein. The reactive oxygen species (ROS) production was measured using the dichloro-fluorescein-diacetate (DCF-DA) probe.19 With the ROS presence, this probe is oxidized and produces the fluorescent compost dichlorofluorescein (DCF). DCF fluorescence is measured in a spectrophotometer with excitation wavelength of 480nm and emission of 520nm. Results were expressed in fluorescence per milligram of total protein. The activity of the antioxidant enzyme superoxide dismutase (SOD) was evaluated by quantifying the ability of SOD to inhibit superoxide radical reaction with adrenaline.20 Absorbance reading was performed at 480nm and data were expressed in units of SOD per milligram of protein. The activity of the antioxidant enzyme catalase (CAT) was evaluated by determining the decomposition rate of H2O2 added to the sample.21 The H2O2 degradation is directly proportional to the CAT activity. Reading was performed in a spectrophotometer at 240nm, and data were expressed in picomoles per milligram of protein.
Measurement of NFκB and TNFα in the gut
Tumor necrosis factor alpha (TNFα) and nuclear factor kappa B (NFκB) in the gut were determined with an ELISA kit for rats (from Abcam and Invitrogen respectively). For the TNFα dosage, the intestinal tissue was lysed, and the test was performed according to the manufacturer's instructions. For the NFκB evaluation, in addition to the tissue lysis, a nuclear extraction protocol (performed according to the manufacturer's instructions) was performed prior to the assay.
Statistical analyses
All values were expressed in means ±S.E.M. Data were analyzed by two-way ANOVA, with diet and supplementation as factors, with Bonferroni post-hoc, using the Prism 5.01 (GraphPad Software). A p-value < 0.05 was considered statistically significant.
Feed intake was lower in HFD-fed groups compared to SD groups (F1,76=525.5, p<0.0001) (Figure 1A). Conversely, feed intake in kJ/rat (energy) was higher in HFD than in SD groups (F1,76<527.7, p<0.0001) (Figure 1B). These findings are explained by the lower palatability of the HFD in relation to SD, however, since HFD is highly energetic, HFD-fed rats acquired more weight and developed a higher visceral adiposity than animals fed with a standard diet as observed in our previous study.22
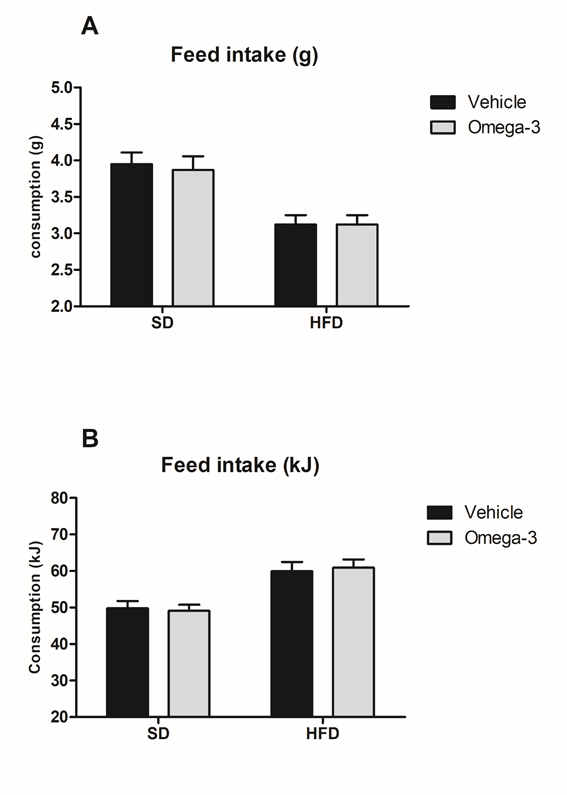
Figure 1 Feed intake. A. HFD groups had a lower consumption of chow (in grams) compared to SD groups. B. Energy intake (kJ) was higher in HFD-fed rats compared to SD groups. Data are presented in mean and S.E.M (n= 8–11/ group). Two-way ANOVA followed by Bonferroni.
HFD, high-fat diet; SD, standard diet.
We evaluated serum lipid profile by measuring total cholesterol, HDL, LDL, and triglycerides. We found that omega-3 supplementation decreased total cholesterol (F1,35=11.61, p<0.01). Figure 2A shows HFD + omega-3 group had lower total cholesterol compared to HFD. HDL did not change among groups (Figure 2B). Also, an effect of omega-3 supplementation was found in cholesterol LDL (F1,30=14.95, p<0.001) and Bonferroni’s test showed that omega-3 supplementation decreased LDL levels in both groups: SD and HFD (Fig. 2C). Regarding triglycerides levels, an interaction between diet and omega-3 treatment (F1,34=14.93, p<0.001) was observed (Figure 2D). Bonferroni post-hoc showed an increase in triglycerides levels in HFD compared to SD group and a significant decrease in HFD + omega-3 compared to HFD, which demonstrated the beneficial effect of omega-3 supplementation in obese rats.

Figure 2 Omega-3 supplementation ameliorates serum lipid profile. A. Total cholesterol; B. Cholesterol HDL; C. Cholesterol LDL; D. Triglycerides. *p<0.05as compared to HFD or SD-vehicle; **p<0.05between SD and HFD with the same treatment. Data are presented in mean and S.E.M (n= 8–11/ group). Two-way ANOVA followed by Bonferroni. HFD, high-fat diet; SD: standard diet.
Data regarding renal and hepatic function are presented in Table 1. A diet (F1,34=7.99, p<0.01) and a supplementation (F1,34=5.06, p<0.05) effects were found in the urea plasma levels. It was found an increase in urea levels in HFD group that was reversed by omega-3 supplementation. In the creatinine levels evaluation, two-way ANOVA showed a significant effect of supplementation (F1,33=13.86, p<0.001) and an interaction between diet and supplementation (F1,33=5.68, p<0.05). HFD+omega-3 had decreased creatinine levels compared to HFD group.
|
Groups |
SD |
SD + omega-3 |
HFD |
HFD + omega-3 |
|
Urea (mg/dL) |
38±3,4 |
35±2,6 |
48±2,0 b |
38±1,4a |
|
Creatinine (mg/dL) |
0,45±0,03 |
0,40±0,04 |
0,59±0,06 b |
0,36±0,03a |
|
AST (U/L) |
197±18,0 |
172±18,0 |
257±11,0 b |
197±12,0a |
|
ALT (U/L) |
28±3,6 |
30±1,7 |
59±4,1 b |
46±2,6a, c |
|
GGT (U/L) |
2,0±0,22 |
2,2±0,20 |
5,4±0,19 b |
4,3±0,21a, c |
|
Alkalinephosphatase (U/L) |
136±12,0 |
165±12,0 |
346±38,0 b |
228±27,0a |
Table 1 Biochemical parameters in the plasma
All values are presented as mean and S.E.M (n=8–11/group). a =compared to HFD; b=compared to SD; c=compared to SD+omega-3. Data were analyzed by two-way ANOVA followed by Bonferroni post-hoc. p<0.05.
HFD, high-fat diet; SD, standard diet; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase
Hepatic function was analyzed by measuring AST, ALT, GGT, and alkaline phosphatase levels. We found a diet (F1,33=7.98, p<0.01) and a supplementation (F1,33=7.93, p<0.01) effects in the AST levels, showing an increase caused by HFD that was reversed by omega-3 supplementation. A diet effect (F1,33=45.23, p<0.0001) and an interaction between diet and supplementation (F1,33=4.70, p<0.05) were found in the ALT levels. Bonferroni’s test indicated that the increase in ALT levels by the HFD was reverted by omega-3 supplementation. The same pattern was observed in the GGT (diet effect (F1,33=179.6, p<0.0001) and interaction between diet and supplementation (F1,33=11.17, p<0.01)) and in the alkaline phosphatase (diet effect (F1,33=30.97, p<0.0001) and interaction between diet and supplementation (F1,33=8.86, p<0.01)) evaluations. Once more, omega-3 supplementation was able to reverse the increase in GGT and alkaline phosphatase levels after an HFD. These results together show that omega-3 plays a beneficial role in HFD-fed rats, improving the overall metabolism by reducing the serum lipid profile values and the markers of renal and hepatic function.
Antioxidant activity and ROS formation was evaluated in the gut. Considering the activity of the antioxidant enzyme SOD (Fig. 3A), a diet effect (F1,35=55.55, p<0.0001), a supplementation effect (F1,35=18.54, p<0.001) and an interaction between these two factors (F1,35=10.91, p<0.01) were observed. Post hoc test evidenced an increase in this enzyme activity in HFD group that was abrogated by omega-3 supplementation. An interaction between diet and supplementation in CAT activity (F1,35=6.394, p<0.05) and a supplementation effect (F1,35=5.37, p<0.05) were also found. Similarly, the increased CAT activity in the gut by HFD was reduced by omega-3 supplementation (Fig. 3B).
When the production of reactive oxygen species (ROS) was evaluated by DCF in the gut, a diet (F1,35=22.89, p<0.0001), a supplementation (F1,35=46.46, p<0.0001) and an interaction effect between these two factors (F1,35=6.58, p<0.05) were found. HFD group produced higher levels of ROS than SD group. Omega-3 supplementation decreased the production of ROS in both supplemented groups (SD+omega-3 compared to SD and HFD+omega-3compared to HFD) (Figure 3C).
Also, a supplementation effect was demonstrated in the evaluation of lipoperoxidation in the gut (F1,34=11.56, p<0.005). Post-hoc comparison between HFD and HFD+omega-3 showed a decrease in malondialdehyde production after omega-3 supplementation (Figure 3D).

Figure 3 Omega-3 supplementation reduces oxidative stress in the gut. A. Activity of antioxidant enzyme Superoxide Dismutase (SOD); B. Activity of antioxidant enzyme Catalase (CAT); C. Production of reactive oxygen species measured by dichloro-fluorescein (DCF); D. Lipoperoxidation measured by thiobarbituric acid-reactive substances (TBARS). *p<0.05 as compared to HFD or SD-vehicle; **p<0.05 between SD and HFD with the same treatment. Data are presented in mean and S.E.M (n= 9–11/group). Two-way ANOVA followed by Bonferroni. HFD, high-fat diet; SD, standard diet.
In the same way, a supplementation effect (F1,34=8.557, p<0.01) was observed in the activation of NFκB in the gut. Bonferroni’s post-hoc showed that HFD+omega-3 group had lower NFkB activation than HFD group (Fig. 4A). For TNFα gut levels, two-way ANOVA showed a diet (F1,35=17.62, p<0.001) and a supplementation (F1,35=6.95, p<0.05) effects. Once more, an increase in TNFα levels by HFD was reversed by omega-3 supplementation in the HFD+omega-3 group (Fig. 4B). These data together show that omega-3 reduces oxidative stress and inflammation in the gut.
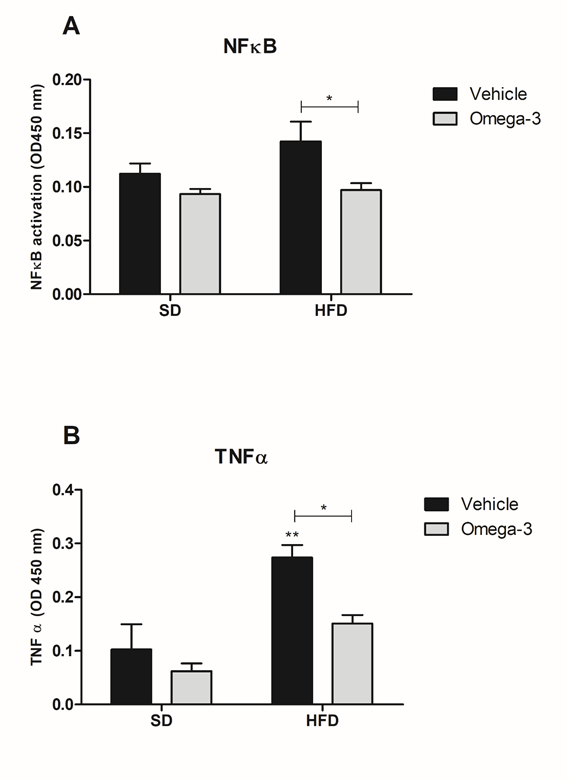
Figure 4 Omega-3 supplementation reduces markers inflammation in gut. A. Measurement of NFκB activation in the gut; B. Evaluation of TNFα production in the gut. *p<0.05 as compared to HFD or SD-vehicle; **p<0.05 between SD and HFD with the same treatment. Data are presented in mean and S.E.M (n= 9–11/group). Two-way ANOVA followed by Bonferroni.
HFD, high-fat diet; SD, standard diet
Oxidative stress and inflammation in obesity generate malfunctioning in several organs and tissues, including the gut.23 The protective effects of omega-3 PUFAs against obesity-associated inflammation have been investigated in many studies.10,12,24 but without focusing on the intestinal tissue. In the gut, the effects of omega-3 were evaluated in the context of inflammatory bowel diseases and more recently, associated with dysbiosis but not associated with obesity. Therefore, it is relevant to study the effects of omega-3 specifically on this tissue. In this sense, we examined the effects of omega-3 supplementation on metabolic parameters such as lipid profile, hepatic function, inflammation, and oxidative stress in the gut of obese rats induced by HFD.
It has been known that obesity is associated with dyslipidemia, increased fasting glucose, and insulin resistance.2,3 The effect of omega-3 in the reduction of the lipid profile is well described, promoting benefits mainly in patients with cardiovascular diseases.25 In a previous study, we showed that 4 weeks of omega-3 supplementation was capable to reduce adiposity index and improve glucose homeostasis and insulin sensitivity in obese rats after 16 weeks of HFD administration.26
In the present study we also demonstrated that 4 weeks of omega-3 supplementation was effective in reducing the plasmatic levels of triglycerides, total cholesterol and LDL in obese rats. This effect might be caused by omega-3 inhibition of lipogenesis and fatty acids oxidation in the liver, and of cholesterol synthesis-related enzymes.27,28 It is important to note that the treatment with omega-3 only started after obesity was established, indicating the promising results of this supplementation even without a reduction in caloric consumption. Most studies suggest that antiobesity effects of omega-3 are predominantly seen when the animals are fed from the start rather than introduced after obesity is already established.
Recently, a systematic review about humans’ studies demonstrated that omega-3 supplementation improved the endothelial dysfunction in individuals with metabolic syndrome and elevated body mass index.29 Yet, there are evidences in the literature indicating that omega-3 supplementation is able to reduce the blood arterial pressure in hypertensive individuals and, also, is able to increase the uncoupling protein-1 (UCP-1) activity, enhancing the energetic deficit in the brown adipose tissue, collaborating to weight loss.30
In the present study, the obesogenic diet induced alterations in the hepatic and renal function markers, indicating a dysfunction in these organs, which was reversed, at least partially, by omega-3 supplementation. Previously, a review by Yang and collaborators evaluated the effects of omega-3 supplementation in non-alcoholic fatty liver disease (NAFLD) patients and observed an improvement in some steatosis hepatic markers with decrease in ALT, AST or GGT levels and/or in the percentage of fat liver.31 However, it is relevant to point that these studies had different doses, formulation type and duration of the treatment. In the present study, we provide additional evidence on the beneficial effects of omega-3 in the liver by using an animal model with controlled dose and time of treatment.
The relationship between obesity and intestinal function has increased in recent years due to the correlation of gut microbiota with the nutrient absorption capacity, changes in intestinal barrier permeability and immune system activation. The human gut is colonized by at least 100trillion of microorganisms that maintain symbiotic relationships with the host. Several studies have demonstrated strong evidence about changes in gut microbiota composition and the etiology of obesity, inflammation, type 2diabetes and NAFLD. Recent data have shown that the gut microbiota regulate the metabolism of the major intracellular antioxidant, glutathione (GSH), in the host organism. Thus, lower levels of GSH can contribute to oxidative stress.31 Another study with obese mice that received probiotics, demonstrated a decrease in Firmicutes/Bacteriodetes ratio and enhanced blood total antioxidant capacity.32
Our study shows that the activity of antioxidant enzymes SOD and CAT were increased in HFD fed animals and decreased in HFD animals supplemented with omega-3. In contrast,33 evidenced that EPA treatment decreased the liver lipid accumulations in obese mice without modifying the oxidative stress biomarkers in this organ. In agreement with our findings, it was demonstrated that the consumption of a high fat and high fructose diet caused increased activity of SOD, CAT, and GPx in the liver of mice.22 Our results can be explained by the increased production of ROS and lipoperoxidation in the HFD group. Higher levels of ROS leads to increased expression of Nfr2, which consequently raises the transcription of antioxidant enzymes to counteract reactive species.34 Therefore, we demonstrated that omega-3 had antioxidant action by reducing ROS and lipoperoxidation in the gut.
The omega-3 fatty acid has further been shown to positively affect eicosanoid production and gene expression, with the ability to inhibit PPARγ and NFκB,35 and have been shown to consistently lower C-reactive protein, tumor necrosis factor (TNF), and interleukins in healthy, non-obese individuals.24 In the present study, omega-3 supplementation reduced NFκB activation and TNFα production in the gut of HFD fed rats. Our findings are consistent with a previous study of Bashir and colleagues36 that showed that fish oil supplementation decreases the levels of NFκB/p65 in the epididymal fat of HFD fed mice, and decreases inflammatory cytokines (TNF-α, IFN-γ, IL-1β, and IL-2) in macrophages isolated from epidydimal fat.36 Costanzo and colleagues37 evidenced that a mixture of Krill Oil (a source of omega-3), the probiotic Lactobacillus reuteri, and vitamin D reduces gut inflammation in mice. The anti-inflammatory mechanism of omega-3 is well known. EPA competes with arachidonic acid for the enzyme cyclooxygenase (COX), thus reducing the synthesis of a series of eicosanoids with a high pro-inflammatory activity.12 In addition, EPA and DHA have other mechanisms to modulate the inflammatory response: by binding to G-protein coupled receptor 120 (GPR120) and reducing the activity of NFκB;38 by inhibiting the NLRP3 inflammasome;39 by activating the peroxisome proliferator-activated receptor gamma (PPAR-γ), which is a transcription factor that acts in an anti-inflammatory manner;40 and through the "stop signs" of the inflammatory response generated by the pro-resolved lipid mediators (SPMs), derived from EPA and DHA, which negatively regulate pro-inflammatory mediators, reducing the magnitude and duration of inflammation, including the gut.41
Gut has vital functions, not only to digest and absorb nutrients, but also to modulate systemic immunity and to prevent bacterial and endotoxin invasion.14 During the development of obesity induced by HFD in mice, the alteration of gut microbiota (dysbiosis) is associated with an increased intestinal permeability, rising the systemic levels of bacteria, including LPS. The last one binds toTLR4 and triggers the activation of many inflammatory pathways leading to the secretion of proinflammatory cytokines, such as TNFα. Overproduction of these cytokines result in intestinal mucosal dysfunction and disruption of tight junction proteins in the gut. This event precedes the development of metabolic endotoxemia, systemic inflammation, and other associated disorders.42–44 However, omega-3 supplementation prevents the ultrastructure alteration of tight junction in a model of ulcerative colitis.16 Furthermore, omega-3 has significant effects on the composition of the intestinal microbiota influencing the return of intestinal homeostasis in situations such as weight gain and obesity, insulin resistance, increased intestinal permeability, and inflammatory bowel diseases.45,46 In our study, we speculate that the anti-inflammatory effects of omega-3 in the gut have occurred by interaction with GPR120 receptor on the membrane surface of enterocytes, thus decreasing NFκB activation and production pro-inflammatory cytokines, such as TNFα, in the intestinal tissue.
In summary, our data support the importance of the supplementation with omega-3 PUFAs, which was able to reverse the effects of HFD in the hepatic and renal function, intestinal oxidative stress and inflammation. Therefore, in this study, we characterized, not only the already known beneficial effects of omega-3 on metabolism, but also its anti-inflammatory and antioxidant action in gut. In this context, our results suggest that omega-3 supplementation may have benefits effects as an adjuvant to reverse the effects of obesity.
We thank Ana Carolina de Moura for reviewing English.
This animal study was performed according to institutional guidelines with the approval of the Institutional Animal Care and Use Committee of the Federal University of Health Sciences of Porto Alegre.
All authors have declared that no conflict of interest exists.
This study was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and PROAP/UFSCPA (Universidade Federal de Ciências da Saúde de Porto Alegre).
Authors' contributions
Gabriela Fernandes Hahn, Michely Lopes Nunes, Luiza Steffens and Jeferson Jantsch performed animal experiments and data collection. Márcia Giovenardi, Renata Padilha Guedes and Marilene Porawski contributed to the study’s conception and design. Bruna Ferrary Deniz, Dinara Jaqueline Moura, Renata Padilha Guedes and Marilene Porawski performed data analysis and interpretation. Gabriela Fernandes Hahn, Jeferson Jantsch and Bruna Ferrary Deniz contributed to drafting of the manuscript.

©2022 Hahn, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.