eISSN: 2373-6372


Review Article Volume 12 Issue 2
1Department of Pediatric Gastroenterology, Bangladesh Institute of Child Health, Bangladesh
2Department of Pediatric Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, India
3Department of Child Health, Faculty of Medicine Universitas Airlangga, Indonesia
Correspondence:
Received: October 25, 2020 | Published: April 27, 2021
Citation: Mahmud S, Sarma MS, Darma A, et al. Neonatal Cholestasis: An Update. Gastroenterol Hepatol Open Access. 2021;12(2):54-66. DOI: 10.15406/ghoa.2021.12.00457
Any infant who is jaundiced beyond two to three weeks of life should be evaluated for neonatal cholestasis. Neonatal cholestasis is defined as accumulation of bile substances in blood due to impaired excretion. The most common causes of cholestatic jaundice in the first months of life are biliary atresia, idiopathic neonatal hepatitis, infections (Cytomegalovirus, herpes simplex toxoplasma, rubella, urinary tract infection, sepsis), endocrine (hypothyroidism), metabolic (Galactosemia, tyrosinemia, neonatal hemochromatosis), genetic (progressive familial intrahepatic cholestasis, Down syndrome, Alagille syndrome) along with many unknown or multifactorial (eg, parenteral nutrition-related) one. Conjugated hyperbilirubinemia, pale stools and dark urine are the cardinal sfeatures of neonatal cholestasis. Newborn screening for biliary atresia by using stool color cards is potentially life-saving and cost-effective. The differential diagnosis of cholestasis is extensive and a systematic approach is helpful to quickly establish the diagnosis. Early recognition, prompt evaluation, timely referrals to the pediatric gastroenterologist/hepatologist and algorithm-based management will improve outcome in neonatal cholestasis. Despite the unavailability of any specific treatments for some causes of neonatal cholestasis, the patient can benefit from nutritional management and early medical intervention.
Keywords: neonatal cholestasis, preterm low birth weight, cystic fibrosis, magnetic resonance cholangiography; endoscopic retrograde cholangiography, liver transplantation
NC: neonatal cholestasis; NTCP: na taurocholate cotransporting polypeptide; OATP: organic anion transporting proteins; BA: biliary atresia; SCC: stool color charts; INH: idiopathic neonatal hepatitis; CC: choledochal cyst; TORCH: Toxoplasma, rubella, cytomegalovirus, herpes simplex; CMV: cytomegalovirus; HSV: herpes simplex virus; PLBW: Preterm low birth weight; PDA: patent ductus arteriosus; PS: pulmonary stenosis; PNAC: parenteral nutrition–associated cholestasis; IUGR: intrauterine growth retardation; SGA: small for gestational age; UTI: urinary tract infection; PIBD: paucity of intrahepatic bile duct; CF: cystic fibrosis; PFIC: progressive familial intrahepatic cholestasis; A1AT: alfa-1 antitrypsin; BASD: bile acid synthetic defect; CLD: chronic liver disease; ALT: alanine transaminase, AST: aspartate aminotransferase, GGT: gamma-glutamyl transpeptidase, ALP: alkaline phosphatase; PT: prothrombine time; INR: international normalization ratio; LB: SPECT: singlephoton emission computer tomography; MRC: magnetic resonance cholangiography; MRCP: magnetic resonance cholangiopancreatography; ERC: endoscopic retrograde cholangiography; ERCP: endoscopic retrograde cholangiopancreatography; IOC: intra-operative cholangiogram; LB: liver biopsy; PE: Portoenterostomy; LT: liver transplantation. SBS: short bowel syndrome; MCT: medium-chain triglyceride; PN: parenteral nutrition; BASM: bilairy atresia splenic malformation; RDA: recommended dietary allowance; AAP: american academy of pediatrics; EPI: expanded Program on Immunization
Cholestatic jaundice is an uncommon but potentially serious problem that indicates hepatobiliary dysfunction in infancy.1 In the 1st 90days of extra-uterine life, neonatal cholestasis (NC) can be defined as conjugated hyperbilirubinemia that occurs when conjugated bilirubin is higher than 1 mg/dl, if the total serum bilirubin is ≤5 mg/dl, or >20% of total serum bilirubin when it is >5mg/dl.2,3 Neonatal conjugated hyperbilirubinemia is pathological and requires evaluation. Jaundice, dark yellow urine staining the diaper with or without pale stools in a new born should be strongly suspected to have NC and should be investigated if persists beyond 14days of life.4
Pathophysiology
The normal process of bile production involves two main processes: uptake of bile acids by hepatocytes from the blood and excretion of bile acids into the biliary canaliculus. Uptake of bile acids from sinusoidal blood is an active process at the sinusoidal membrane of the hepatocytes. Na taurocholate cotransporting polypeptide (NTCP) and organic anion transporting proteins (OATP) are the two main receptors involved in the uptake of conjugated bile acids by the liver cells. These receptors are also responsible for the transport of other anions like drugs and toxins through the hepatocellular membrane. In newborn infants, the biliary system is both structurally and functionally immature making them more susceptible to cholestasis. In hepatitis (of various causes) and sepsis, there is down regulation of the NTCP and OATP receptors resulting in decreased bile production and cholestasis. Various genetic defects in the transporter proteins have been recognized in familial cholestasis syndromes, eg, mutation of bile salt export pump (BSEP) gene in progressive familial intrahepatic cholestasis (PFIC) type 2, defect in the multidrug resistance protein 3 in PFIC type 3. 3
Incidence
In the west, NC affects 1 in 2500 infants. In India, it constitutes 19% to 33% of all chronic liver disease (CLD) in children.5–7 The referral pattern of NC in Asia still continues to be concerning due to poor primary health care, lack of knowledge and illiteracy. Delayed referrals precludes portoenterostomy (PE) in biliary atresia (BA), leads to secondary biliary cirrhosis and enlists them for primary liver transplantation (LT). Potentially lethal but correctable disorders such galactosemia, tyrosinemia and neonatal hemochromatosis are missed and are detected when complications have ensued. Measures to improve the referral pattern in various countries include stool card at community level and newborn metabolic screening at birth. Idiopathic neonatal hepatitis (INH) has been reported to have an incidence of 1 in 4,800 to 9,000 live births. Whereas biliary atresia (BA) ranges from 1 in 6,000 to 18,000 live births. 1–4,8
Classification
NC can be classified into extrahepatic and intrahepatic causes based on the anatomic location of the pathology. Among extrahepatic causes, BA and choledochal cyst (CC) are the examples while common intrahepatic causes include INH, infections, endocrine and other metabolic disorders (Figure 1). 8
Etiology
BA (25%–35%), genetic disorders (25%), metabolic diseases (20%), and alfa-1 antitrypsin (A1AT) deficiency (10%) are the commonly identifiable conditions in United States (US). In older series, INH was the most common cause of NC. However, with the more advanced diagnostic methods and addition to discovery of specific etiologies, the incidence of INH has decreased substantially. Premature infants and in those who have short bowel syndrome (SBS) or intestinal failure and receiving parenteral nutrition (PN) for more than 2 to 4weeks, commonly develops parenteral nutrition–associated cholestasis (PNAC) (Tables 1 & 2). 2
|
Intrahepatic |
Obstructive |
|
Idiopathic Neonatal Hepatitis a) Viral infection b) Herpes simplex c) Cytomegalovirus d) Toxoplasma e) Parvovirus B19 Other |
a) Biliary atresia b) Choledochal cysts c) Neonatal gallstones/biliary sludge d) Caroli disease e) Neonatal sclerosing cholangitis f) Inspissated bile plug syndrome |
|
Bacterial infection a) Sepsis b) Urinary tract infection |
|
|
Genetic/Metabolic disorders a) Galactosemia b) Tyrosinemia c) PFIC d) Alagille syndrome e) Bile acid synthetic defects f) Alfa-1 antitrypsin deficiency g) Neonatal hemochromatosis |
|
|
Endocrine disorders a) Hypothyroidism b) Hypopituitarism |
|
|
Toxic a) Drugs b) Parenteral nutrition Others a) Preterm low birth weight |
|
Etiologic Factor |
India N=420 (%) |
Bangladesh N=80 (%) |
|
Biliary atresia |
126 (30) |
30 (37.4) |
|
Choledochal cyst |
21 (5) |
2 (2.5) |
|
Infections |
75 (17.8) |
20 (24.9) |
|
Metabolic |
50 (11.9) |
5 (6.2) |
|
Idiopathic |
144 (34.3) |
21 (26.5) |
|
Ductal paucity |
4 (1.0) |
2 (2.5) |
Table 2 Summarize the etiologic Profile of NC in India7 & Bangladesh9
History
Obtaining a detailed prenatal and infant history is fundamental.1 The infant’s family history may provide important information, such as consanguinity & miscarriage (genetic disease), while the obstetric history like fever with rash may reveal maternal infection (TORCH infection, hepatitis B) or cholestasis of pregnancy (which may be associated with PFIC).10 History of pre-term low birth weight (PLBW), intrauterine growth retardation (IUGR), small for gestational age (SGA) may suggest intrahepatic cholestasis (congenital infections) whereas a term gestation, normal weight baby may favor of BA.8,10 Details of feeding history (feeding intolerance & convulsion in Galactosemia) should be noted as well as the timing of the first bowel movement, because delayed passage of meconium can be seen in patients with cystic fibrosis and congenital hypothyroidism.1 Pale stool (usually persistent in BA & intermittent in neonatal hepatitis) and history of onset of jaundice is also crucial. Yachha, et al. showed that the age of onset of jaundice in BA was 3-12 days and that of hepatocellular causes was 16-24 days.12 However, the mean age of presentation to a tertiary care center was 2.8–3.9 months compared to the desired age of evaluation, that is 4-6 weeks.12–14
Jaundice
Jaundice, dark urine and/or pale stool is suggestive of cholestasis (Figure 2).4 As the indirect bilirubin decreases, jaundice may decrease over the first weeks of life, thus giving a false impression that the jaundice is resolving. Deep jaundice suggests associated hemolysis, renal failure or dehydration in an underlying parenchymal liver disease. In terminal stages, jaundice may worsen. Mild jaundice with overwhelming poor synthetic functions may be seen in early cirrhosis such as tyrosinemia and neonatal hemochromatosis. 4,10
Growth & development
In spite of jaundice, babies with BA appear well and have normal growth and development, and this leads to parents and physicians underestimating the seriousness of the problem.4 Many health care professionals also have a misconception that all well babies with icterus have physiological jaundice (which is unconjugated and associated with normal urine color). On the other side, normal growth usually impaired in neonatal hepatitis.
Stool color
Acholic stools is suggestive of cholestasis (intrahepatic or extrahepatic) (Figure 3). Persistently acholic stool should alarm for extrahepatic biliary obstruction. Lack of meconium at birth and acholic stools from day one is high suspicious of BA. On the other hand, the presence of pigmented stools has negative predictive value, suggests patency of the extra-hepatic biliary tree and makes BA unlikely. In the early course of BA, stools can erroneously be interpreted as intermittently pigmented and therefore, it is important that stool color should serially assessed by the physician. Intermittently pale and pigmented stools are seen in intrahepatic causes that have fluctuations in liver activity and inflammation. They may be seen infectious, metabolic or idiopathic causes.10
Stool color card
For actual diagnosis of cholestasis cases, history of pale or acholic stools along with observe the stool pigment is highly recommended. It is well recognized that parents and health care professionals assess stool pigmentation subjectively and abnormally pale stools are frequently misinterpreted as normal.1 Acholic stools were correctly identified only by 63% of health care providers.15 Stool color charts (SCC) much beneficial in review of history and bed side examination to identify the intermittent or persistent pale stool (Figure 4). In Taiwan, use of a SCC proved to be effective with 95.2% sensitivity for pale stools.16 Home-based screening for BA with a stool card proved cost effective in Canada.17 The sensitivity, specificity, and positive predictive value of pale stools for the detection of BA before 60 days as determined by a color-coded SCC was noted to be 89.7%, 99.9% and 28.6%, respectively.16
Dark urine
Water soluble conjugated bilirubin presented as a dark urine but is not pathognomonic (Figure 5).10 This urine color not routinely observed by parents as well as not examined by physicians.
Organomegaly and mass
Physical examination of the cholestatic infant may reveal universally has hepatomegaly.8 Splenomegaly is observed in majority, irrespective of etiology. Spleno-hepatomegaly is a feature of storage disorders such as Gaucher’s and Niemann-Pick disease. Absence of spleen does not rule out BA as <10% may be associated bilairy atresia splenic malformation (BASM) syndrome. In these cases, splenomegaly is not palpable due to splenunculi or ectopic spleens. A palpable mass in the right upper quadrant may indicate a palpaple CC.10
Facial Dysmorphism
Syndromic and chromosomal (Down, Alagille etc.) disorders may presented with dysmorphic facial features (Figure 6).10 Rare syndromes such as Zellweger, Smith-Lepli-Opitz are also associated with dysmorphism. 2
Ocular Manifestations
Ocular findings are also very important. Posterior embryotoxon and drusen can be present in Alagille syndrome, optic nerve hypoplasia in pan-hypopituitarism, chorioretinitis in cytomegalovirus (CMV) infections and cataract in rubella or galactosemia (Figure 7). 10
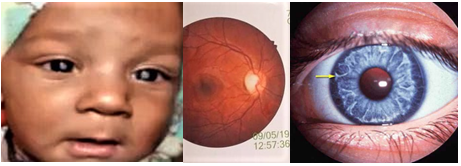
Figure 7: Cataract (Left), Cherry red spot (Middle) and Posterior embryotoxon (Right) (Picture was taken with permission).
Cardiac findings
Cardiac evaluation should necessary in some cases. Peripheral pulmonary stenosis or other cardiac anomalies in Alagille syndrome, dextrocardia in BA and patent ductus arteriosus (PDA) or septal defects in congenital infections.10
Other features
Other than lethargy, poor feeding, and seizures in a cholestatic neonate there are features that must always alarm the pediatrician warranting early workup. These are uncorrectable coagulopathy, hypoglycemia, ascites, family history of early infantile sib deaths, maternal history of stillbirths, abortions, associated fever, anemia or respiratory distress. As a result of liver failure, coagulopathy may also present that indicating either a severe metabolic hepatic disorders (as in respiratory chain deficiency disorders) or cirrhosis and severe liver disease (as in neonatal hemochromatosis) (Table 3).10
|
Subjects |
Extrahepatic cholestasis |
Intrahepatic cholestasis |
|
Sex |
More common in female |
More common in male |
|
Incidence |
Sporadic |
Familial (20%) |
|
Gestational age |
Term, Normal weight baby |
PLBW, IUGR, SGA |
|
Congenital anomaly |
Usually present |
Usually absent |
|
Pale stool |
Persistent (BA) |
Intermittent |
|
Jaundice |
Usually mild to moderate |
Usually severe |
|
Splenomegaly |
Usually present in later stage |
May present in initial stage |
|
Thriving & activity |
Well & active |
Not well with poor activity |
|
Facial dysmorphism |
Uncommon |
May present |
|
Eye findings |
Usually absent |
May present |
Table 3 Difference between extrahepatic and intrahepatic cholestasis1-14
Specific diseases
Biliary atresia
BA is an idiopathic fibrosing cholangiopathy of unknown etiology that leads to complete obstruction of the extrahepatic bile duct during the first few months after birth, progressive biliary cirrhosis, and eventual death if left untreated. It accounts for approximately one-third of the cases of NC. It is the most important differential diagnosis in NC because it should be recognized and treated before the infant reaches the 60days of age.2,10 There are two clinical forms of BA: 80% of infants have isolated atresia without other congenital malformations and are labeled as having the perinatal or so called acquired form; they show normal early growth, and develop or have persisting jaundice and acholic stools at approximately 3 to 6 weeks of age. The remaining 20 % of infants who have BA have congenital malformations, including BASM syndrome or other isolated major congenital malformations (splenic and hepatic vascular anomalies, situs inversus, congenital heart disease, etc.), and are labeled as having the so-called fetal/embryonic form. Mahmud et al.9 (37.5%) and Nahid et al.18 (31.6%) stated that BA was the most common etiology of NC of Bangladeshi children. These infants may appear jaundiced at birth and and remain so.3,10
Children with BA are usually born after a normal pregnancy, show normal early growth, generally have prolonged jaundice, and develop acholic stools at approximately 3 to 6 weeks of age (Figure 8). Then they develop failure to thrive, pruritus and coagulopathy, while physical examination indicates hepatomegaly and splenomegaly. Ascites and other features of cirrhosis may be seen late in the disease process. 2,3,10
Choledochal cyst
CC is a congenital dilatation of the biliary tree which is benign but can be complicated by cholestasis and cholelithiasis. Sometimes patients have cholangitis and present with fever, elevation of the GGT (gamma-glutamyl transpeptidase), and direct hyperbilirubinemia. Ultrasonography can often differentiate between CC and BA as the bile ducts are typically dilated or cystic and the gall bladder is not atretic. After ultrasonogram, magnetic resonance cholangiopancreatography (MRCP) is the ideal tool for the diagnosis. Early diagnosis and intervention in CC through excision was a safe procedure with fewer complications. 1. Mahmud et al.9 (6.3%) and Nahid et al.18 (9.5%) stated that CC was the 2nd most common etiology of extrahepatic cholestasis of Bangladeshi children.
Idiopathic neonatal hepatitis (INH)
INH also known as giant cell hepatitis, accounts for approximately one-third of the cases of NC. It is diagnosed by the presence of the classic pathological findings and the absence of any identifiable cause of cholestasis (Figure 9). There are two different categories: sporadic cases and familial cases that could likely suggest an undiagnosed genetic or metabolic disease. These infants usually have low birth weight. Jaundice is present within the first week of life. Acholic stools are usually absent unless there is severe cholestasis. INH was the common etiology in NC of Bangladeshi infants according to Nahid et al.18 (31.6%) and Karim et al.19 (24.2%) study. On physical examination, liver is enlarged and firm in consistency. Serum bilirubin and transaminases are mildly elevated. Liver biopsy (LB) usually shows lobular disarray with hepatocellular swelling (ballooning), focal hepatic necrosis and giant cell transformation with evidence of extramedullary hematopoiesis. Management is usually supportive with nutritional support, vitamin supplementation and treatment of complications of cholestasis. Prognosis is variable with sporadic cases having very good prognosis with 90% resolution by age 1 year and relatively poor prognosis in familial cases suggesting some inborn errors. 3,10
Infections
CMV the most common congenital infection, affects 1% to 2% of newborns. Most infected newborns are asymptomatic; unfortunately 5% to 10% of the patients have a myriad of clinical symptoms that include low birth weight, microcephaly, periventricular calcifications, chorioretinitis, and deafness. Hepatosplenomegaly and direct hyperbilirubinemia are the most prominent liver-related problems (Figure 10). The diagnosis of congenital CMV is confirmed by culture or PCR from the nasopharynx, saliva, blood, or urine soon after birth. Urine CMV culture or CMVDNA detection by PCR is presently used for the diagnosis. The immunoglobulin M (IgM) CMV-specific antibodies can be monitored but are of limited value and may be less sensitive. Evidence of recent CMV infection at the time of diagnosis of BA has been reported but a role for CMV in the etiology of BA remains unproven. Rubella, toxoplasma, and herpes virus also can present with neonatal cholestasis, coagulopathy and growth restriction. Out of 19 cholestatic TORCH infections, CMV (68.4%) were the most commonest followed by CMV with herpes-simplex virus (HSV) co-infection (21.1%) and Toxoplasmosis (10.5%) among Bangladeshi infants.9 Obtaining a good maternal history and discussing with the obstetrician and neonatal intensive care team about placental abnormalities can help with directing the workup for an infection.1,4
Hypothyroidism
Few reports in pediatric literature describe cholestatic liver disease in congenital hypothyroidism. History of delayed meconium pass, prolong neonatal jaundice and constipation are the key for suspecting hypothyroidism. Coarse facis, Hypo activity with harsh cry may suggest thyroid hormonal deficiency (Figure 11).1 In a study of Bangladesh, out of 62 cholestatic infants, Karim et al.19 was observed 2.3% hypothyroidism with Down syndrome and 2.3% hypothyroidism with CMV infection. From another study Nahid et al.18 reported the near similar results among 39 intrahepatic cases, isolated hypothyroidism in 10.2% and hypothyroidism with Down syndrome in 2.5% cases. Several studies from India observed that marked improvement with levothyroxine supplementation in NC with hypothyroidism. The newborn screen is designed to detect high levels of thyroid stimulating hormone (TSH); hence, in cases of central hypothyroidism, this can be missed and repeating a blood TSH, free T4, and T3 may be helpful. 1
Galactosemia
Galactosemia is an autosomal recessive disorder that occurs in 1 in 50,000 live births. Out of 80 cholestatic infants, Two (2.5%) cases of galactosemia were found in a study of Bangladeshi infants.9 A deficiency of the enzyme galactose-1-phosphate uridyl transferase results in defective metabolism of galactose (Figure 12). Newborn screening for galactosemia is performed in most countries, thus identifying the majority of infants before they become symptomatic. However, infants who have galactosemia may present with failure to thrive, vomiting, diarrhea, cataracts,
E. coli septicemia, jaundice and cholestasis, hepatomegaly, ascites, or hypoglycemia. Treatment of galactosemia involves dietary avoidance of all foods that contain galactose and lactose.2
Progressive familial intrahepatic cholestasis (PFIC)
PFIC is a group of autosomal recessive disorders that show progressive intrahepatic cholestasis. Among all cholestatic infants, only 1 (1.2%) case of PFIC was found in Mahmud et al.9 and Nahid et al. study.18 PFIC-1 is caused by mutation in the FIC1 gene and is the original Byler disease described in the descendants of an Amish American family. The FIC1 gene is expressed in the canalicular membranes and present with episodic cholestasis in the first month of life. Diarrhea, pancreatitis and deficiency of fat-soluble vitamins are seen. Serum GGT levels are normal. LB shows bile duct paucity. Management is mostly supportive. Surgical methods like ileal exclusion, partial external biliary diversion have been tried. Cirrhosis is seen by end of first decade of life and liver transplantation is needed with hepatic decompensation and is usually needed around the second decade of life. PFIC-2 is caused by a defect in the canalicular bile salt excretory pump (BSEP). Clinical presentation is similar to PFIC 1 except for the absence of pancreatitis in this condition. LB shows more inflammation and electron microscopy shows amorphous bile. Management is again supportive. Prognosis is worse, with patients requiring LT in the first decade of life. PFIC-3 is caused by a defect in the canalicular phospholipids transporter, MDR3. Clinical presentation is similar to PFIC-1 but is delayed until early adulthood. There is a history of cholestasis of pregnancy in the mother. GGT is markedly elevated and bile analysis shows high bile acid to phospholipid ratio. LB may mimic BA but the biliary tract is patent. Treatment is mostly supportive and prognosis is variable. 3
Alagille syndrome
Alagille syndrome is an autosomal dominant multisystem disorder characterized by a paucity of intralobular bile ducts (PIBD) and occurring in approximately 1:70,000-100,000 live births.
Almost all patients (90 %) have a mutation in the JAGGED 1 gene that encodes a ligand in the Notch signaling pathway, while others have less frequent NOTCH-2 receptor mutations. Patients with Alagille syndrome have a characteristic face with triangular shape, broad forehead, deep-set eyes, small pointed chin, and bulbous nose; skeletal anomalies including butterfly vertebrae, curved phalanges, and short ulna; cardiac anomalies, most commonly peripheral pulmonary stenosis; ocular anomalies such as posterior embryotoxon and optic nerve drusen (Figure 7). Other findings include renal abnormalities such as ectopic kidney; mental retardation and developmental delay; growth retardation, short stature and pancreatic insufficiency. Infants usually present with neonatal cholestasis. It may be difficult to differentiate from BA because in some cases initial LB may show bile duct proliferation, and the characteristic facies may not be evident in the neonatal period. Management is mostly supportive with adequate nutritional intake and treatment of pruritus. Supplementation of fat-soluble vitamins and pancreatic enzymes is needed. The outcome of Alagille syndrome is largely dependent on the individual’s clinical manifestations, especially the severity of the cardiac and renal lesions. More than half of children presenting with NC progress to cirrhosis and require LT by age 10. 10
Alpha-1-antitrypsin (A1AT) deficiency
A1AT deficiency is an autosomal recessive disorder, which is commonly seen in Northern European descent and extremely unusual in Asians. It’s affecting approximately 1 in 2,000 live births. Only 1 case of cholestatic infant with A1AT deficiency was found in Karim et al.19 study from Bangladesh. Affected individuals have a misfolded A1AT protein that fails to be secreted normally by the hepatocyte, leading to decreased A1AT activity in the blood and lungs and excess retention in hepatocytes.2 The deficiency is caused by mutations in the gene found on chromosome 14.3 Approximately 10% to 15% of neonates with this condition will present with cholestasis and a combined picture of hepatocellular injury and obstruction with elevation of the ALT, AST, GGT, and ALP. The cholestasis is usually severe and the presence of acholic stools may present a challenge because of the resemblance to BA. Although some patients may develop cirrhosis early on, jaundice clears in most patients by 4 months of age. 1 Serum A1AT assay, LB and genetic testing are the key for diagnosis. Giant cell hepatitis with bile ductular proliferation are common whereas PIBD may be found later on.8 There is no specific treatment for A1AT deficiency. Children who develop cirrhosis and liver failure may require LT.2
Bile acid synthesis disorders (BASDs)
More than 14 enzymes are involved in the synthesis of bile acids. BASDs are rare, but in many cases are treatable forms of cholestasis. Not all of the infants with the genetic abnormalities leading to BASD present with cholestasis and jaundice; some may have a more indolent presentation later during childhood. These conditions often present with normal or low GGT. Total serum bile acids are usually low, in contrast to other cholestatic disorders. Molecular techniques then identify the specific mutations in genes encoding the enzymes responsible for bile acid synthesis. Treatment with the end products of bile acid synthesis, cholic acid and chenodeoxycholic acid, is often curative for several patients. 1
Sepsis
Septic induced cholestasis is a kind of hepato-cellular cholestasis that occured during or after sepsis caused by biliary flow obstruction. The mediators that trigger released of cytokines are bacterial endotoxin and lipopolysacharides (LPS). Mostly preterm low birth weight (1500-2499 gram) with immature immune system are more susceptible to infection. Majority of neonatal sepsis are caused by gram negative bacteria like Acinetobacter calcoaceticus followed by Enterobacter aerogenes and Klebsiella pneumoniae.11 Unstable respiratory rate lethargy, poor feeding, hypotonia or seizures are the most frequently found signs in sepsis. In an acutely ill infant sepsis, shock, heart failure, hypopituitarism, and metabolic disorders such as galactosemia or tyrosinemia should be evaluated promptly.10
Urinary tract infection
As a part of sepsis, urinary tract infection (UTI) can also causes NC in the post neonatal period and patients may present as isolated cholestatic jaundice without other manifestations of infection. Thus, a strong suspicion of systemic infections especially UTI should be kept in mind with NC.20 Out of 62 cholestatic infants, 5 (8%) cases of UTI were found in a study of Bangladesh.19
Preterm infants with cholestasis
Cholestasis is a common finding in very low birth weight infants and is multifactorial in etiology. The biliary tract is structurally and functionally immature in these infants and can be exaggerated by further cholestatic insults such as perinatal asphyxia, poor enteral feeding, parenteral nutrition, drug toxicity and sepsis.3 Fish oil-based lipid emulsions are composed of omega-3 fatty acids is effective in promoting recovery from cholestasis in preterm infants. The management of cholestasis in preterm infants should include the discontinuation of parenteral nutrition as soon as possible and the promotion of enteral feeding (also trophic feeding) that enhances bile flow, gallbladder contraction, and intestinal motility. Treatment with UDCA may be given although there is no evidence of its efficacy in this specific pathology.10
Parenteral nutrition associated cholestasis
Overall, 18% to 67% of infants who receive prolonged courses of PN (longer than 14days) develop liver injury and cholestasis. The incidence of PNAC is correlated inversely with birth weight and directly with duration of PN therapy. Infants who have sepsis, bacterial overgrowth of the small intestine and intestinal failure are at increased risk for developing PNAC.2 A novel lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil (SMOFlipid) with reduced omega-6 fatty acids, increased omega-3 fatty acids, and enriched in vitamin E was found to decrease the GGT serum level, oxidative stress, retinopathy of prematurity and bronchopulmonary dysplasia. Taurine supplementation seems to offer a significant degree of protection against PNAC, particularly in patients with necrotizing enterocolitis or severe prematurity.10
Investigations
Liver function test
The most important initial investigation is the measurement of fractionated serum bilirubin levels. As aforementioned, infants who have cholestasis will have >1mg/dL conjugated bilirubin when the total bilirubin is <5 mg/dL or >20 % of the total bilirubin level if total bilirubin is >5mg/dL. Recently, it has been reported that in the first 4days of life, the cutoff for elevated conjugated bilirubin may be >0.8mg/dL and from 8 % to 10 % of the total bilirubin. 2,10 It has also been suggested that in the first 14days after birth, the cutoff for elevated conjugated bilirubin may be >0.5mg/dL, and >2mg/dL for conjugated bilirubin.2,10 Serum transaminases, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), are sensitive indicators of hepatocellular injury but are neither specific nor of prognostic value. They are important to indicate resolution in a resolving hepatitis. Elevated levels of ALP can be found in biliary obstruction but also in the course of bone and kidney diseases. GGT is an enzyme in the biliary epithelium whose increase is strongly associated with cholestatic disorders like BA, CC (extra-hepatic obstructions), neonatal sclerosing cholangitis, A1AT deficiency and Alagille syndrome. However, a low or normal GGT out of proportion to the degree of cholestasis suggests the presence of PFIC type 1, PFIC type 2, 4, 5 or 6 and an inborn error of bile acid synthesis.2,4,10
X-ray & echocardiogram
Chest X-ray & echocardiogram should be performed when cardiac anomalies are suspected (in case of murmur). In fact, up to 24% of patients with Alagille syndrome and a subset of BA patients have structural heart disease. Added skeletal survey when Alagille syndrome (butterfly vertebrae) is suspected (Figure 14).2,10
Abdominal ultrasonography
An abdominal ultrasonography examination should be obtained as part of the early evaluation of a cholestatic infant to assess liver structure, size, and composition; to evaluate for the presence of ascites; and to identify findings of an extrahepatic obstructive lesion (CC, mass, gallstone, and sludge).2 Abdominal ultrasonography findings described in BA include the triangular cord sign (cone-shaped fibrotic mass >4mm anterior to right branch of portal vein), abnormal gallbladder morphology (not visualized or length <1.9cm or lack of smooth/complete echogenic mucosal lining with an indistinct wall or irregular/lobular contour), poor contractility (<60%) of the gallbladder 2hours after oral feeding (Figure 15). A distended gall bladder, however, does not rule out a proximal BA with a distal patent bile duct and mucus filled gallbladder.2,4 It is recommended that ultrasound should be done after 4hours of fasting. Ultrasound can also detect polysplenia or asplenia, interrupted inferior vena cava, preduodenal portal vein, and situs inversus; all of these conditions would strongly suggest BA splenic malformation syndrome and other laterality defects. CBD dilation is not seen in BA and suggests a distal obstruction or a forme fruste CC. In normal infants CBD is seen with difficulty (<2mm diameter). Hence non-visualisation of CBD is not synonymous with BA. Cyst at porta may be seen in BA and can be differentiated from CC by the absence of intrahepatic biliary dilatation and rudimentary gall bladder (Figure 16). 2
Hepatobiliary scintigraphy
It is carried out using Technetium-99 m-labeled hepatobiliary-immuno-di-acetic acid (HIDA) derivatives (Tc-99 IDA). For enhancing the study, a pretreatment with oral phenobarbitone (5mg/kg/d) or ursodeoxycholic acid (10-15mg/kg) for at least 3 days previously. Presently ursodeoxycholic acid is more preferred than phenobarbitone due to adverse CNS effects (sedation, irritability). The best resolution is achieved if the patient is administered a pretreatment with phenobarbitone (5mg/kg/d) for at least 3days previously. Serial images are taken for up to 24h or until gut activity is visualized. In a healthy infant, the injected radioisotope is taken up by the hepatocytes, secreted into the biliary system, and then excreted into the small intestine within 24hours. Care should be taken not to erroneously interpret urinary contamination over abdomen as intestinal excretion. Hence babies are covered with diapers during the test. Slow uptake of the injected radioisotope or non-visualization of the liver with persistence of the cardiac pool suggests hepatocellular dysfunction, whereas non-visualization of the radioisotope in the small intestine from 4 to 24 hours suggests either bile duct obstruction or the severe inability of the hepatocyte to secrete (Figure 17).2,4,10 The sensitivity of scintigraphy for BA is relatively high (83 %–100 %); however, its specificity is low (33 %–80 %).21,22 A recent meta-analysis of 81 studies has shown a pooled sensitivity and specificity of 98.7% and 70.4%, respectively.23 However, when an acholic stool has been seen by an experienced observer scintigraphy adds little information. In fact, since hepatobiliary scintigraphy is expensive, time consuming and poorly specific, many centers do not routinely use this test in the evaluation of cholestatic infants because it may delay the diagnostic evaluation without providing definitive diagnostic information.24 However, others think that it still has a role where the liver biopsy is unavailable or ambiguous and in the evaluation of preterm infants.2 Its also useful in the diagnosis of uncommon causes like spontaneous perforation of bile duct. Recently it has been suggested that singlephoton emission computer tomography (SPECT) can improve the specificity (81.1 %) of hepatobiliary scintigraphy allowing better bowel visualization compared to planar images.25 However, only two studies have evaluated the SPECT method and further studies are needed.10,26,27
Magnetic resonance cholangiography (MRC)
A few reports suggested that Magnetic resonance cholangiography (MRC) can be used to assess the biliary tract. Non- visualization of the common bile duct and the presence of a small gallbladder have been noted in BA.28 The diagnostic value of 3-dimensional Magnetic resonance cholangiopancreatography (MRCP) for BA reported sensitivity 99% but specificity 36%.29 Now a days, insufficient data are available for MRC evaluation of cholestatic infant and further studies are required to recommend routinely this modality.8,28,29 MRCP in an infant has technical difficulties. Spatial resolution is poor in small infants, possible movement artefacts and most importantly absent bile flow in a non-dilated biliary system makes interpretation difficult.
Endoscopic retrograde cholangiography (ERC)
The role of endoscopic retrograde cholangiopancreatography (ERCP) in the diagnosis of BA has been studied by various groups. Although ERCP has proved effective with high positive and negative predictive values for BA (sensitivity 86%–100%, specificity 87%–94%, positive predictive value 88%–96%, negative predictive value 100%), ERCP requires an experienced endoscopist, specific pediatric duodenoscope (not readily available at many centers), and a general anesthesia.1,30,32 Difficult canulation may be misinterpreted as non-visualisation of CBD. As a result ERC is of limited usefulness for the evaluation of NC in most centers. 24
Liver biopsy (LB)
Percutaneous LB in early infancy under local anesthesia and sedation is a safe procedure, if performed by competent physicians.4,33 LB is the single most definitive investigation in the evaluation of NC. In several single center studies, a diagnosis of BA was correctly suggested by LB histological findings in 90 to 95% of cases and avoid unnecessary surgery in patients with intrahepatic disease (Figure 19).1,10,34 Rastogi et al. also reported that sensitivity, specificity and accuracy of liver biopsy in the diagnosis of BA are 89-99%, 82-98% and 60-95%, respectively.4,35 The diagnostic histologic appearances of BA include bile duct proliferation, bile plugs in the portal tract bile duct, portal tract edema and fibrosis (Figure 20). Liver histology also useful for the diagnosis of other specific conditions, such as A1AT deficiency, some metabolic liver diseases, Alagille syndrome, neonatal sclerosing cholangitis, and viral infection (CMV or herpes simplex).10 Presence of steatosis indicates metabolic liver diseases. Rarely LB may yield pathognomic findings of storage disorders.

Figure 19: Biliary atresia. Significant ductular proliferation, cholestasis and portal fibrosis (H&E x 100).
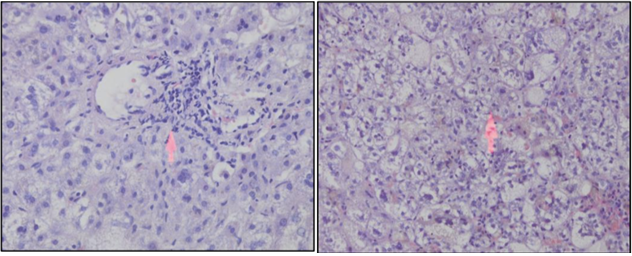
Figure 20: Intrahepatic cholestasis with inflammed cell infiltration (left) and hepatocute degeneration (right).
Intraoperative cholangiogram
Intra-operative cholangiogram (IOC) remains the gold standard for diagnosis of BA.1,4 It is a mini-laparotomy to delineate the biliary anatomy and localize the area of obstruction. The surgeon should be prepared and capable of performing a PE for BA or CC corrective surgery during the same surgical session if these lesions are found on cholangiography.2 Presently many centers are considering neonatal laparoscopic cholangiography for assessing patency of extrahepatic bile ducts (Figure 19 & 20).
Accurate test for specific condition
To establish a definite diagnosis, specific tests necessary (Table 4).
|
Disease |
Tests |
|
Biliary atresia Choledochal cyst INH Congenital infections |
USG of HBS, HIDA scan, Liver biopsy USG of HBS, MRCP Exclude other causes TORCH screening, Urinary CMV-PCR |
|
Sepsis Urinary tract infection |
CBC, CRP, Blood C/S Urine R/M/E & C/S |
|
Congenital hypothyroidism |
Free T4, T3 & TSH |
|
Pan-hypopituitarism
Galactosemia |
Free T4, T3 & TSH, Cortisol (early morning), MRI brain of brain Non-glucose reducing substance (NGRS), Serum galactose-1 phosphate uridyl transferase (GALT) assay, genetic testing |
|
Tyrosinemia |
Serum alfa-fetoprotein (AFP), Urine & serum succinylacetone assay |
|
Hemochromaetosis |
Serum ferritin, buccal mucosal biopsy |
|
PFIC Alagille syndrome
|
Serum GGT, genetic testing X-ray chest & vertebra (both view), Echocardiogram, genetic testing |
|
A1AT deficiency |
Serum Alpha-1-Antitrypsin assay, genetic testing |
|
Cystic fibrosis |
Sweat chloride test, genetic testing |
|
Hereditary fructose intolerance |
Aldolase B enzyme assay in Liver biopsy sample (fructose challenge test now obsolete) |
|
Bile acid synthesis defects
PLBW |
Serum GGT, Urine & serum bile acid analysis, genetic testing Liver function tests |
|
|
|
Management
Medical management of cholestasis is mostly supportive and does not alter the natural course of the disease. It is aimed mostly at treating the complications of chronic cholestasis like pruritus, malabsorption and nutritional deficiencies and portal hypertension. 3
Nutritional management
Most infants with NC are underweight and will need nutritional support (Table 5). The goal is to provide adequate calories to compensate for steatorrhea and to prevent/treat malnutrition. The calorie requirement is approximately 125% of the recommended dietary allowance (RDA) based on ideal body weight.8 In breastfed infants, breastfeeding should be encouraged and medium-chain triglyceride (MCT) oil should be administered in a dose of 1-2mL/kg/d in 2-4 divided doses in expressed breast milk. In older infants, a milk-cereal-mix fortified with MCT is preferred. Adding puffed rice powder and MCT to milk can make feeds energy-dense. Essential fatty acids should constitute 2-3% of the energy provided. Vegetable protein at 2-3 g/kg/d is recommended (Figure 21).4,36
|
Vitamins |
Routes |
Dose |
|
Vitamin A |
Oral I/M |
5,000-25,000IU/day 50000IU stat then 10000IU monthly |
|
Vitamin D |
Oral I/M |
400-1200 IU/day 2 lac IU/1ml vial, 30000IU monthly (60000 IU if rickets) |
|
Vitamin E |
Oral I/M |
15-25IU/kg/day 50-200mg monthly |
|
Vitamin K |
Oral
I/M or S/C or I/V |
2.5mg twice/week to 5 mg/day 2-5mg |
|
Water soluble vitamins |
Oral |
1-2 times of RDA |
|
Calcium |
Oral |
20-100mg/kg/day |
|
Phosphorus |
Oral |
25-50mg/kg/day |
|
Zinc |
Oral |
1mg/kg/day |
|
Magnesium |
Oral I/V |
1-2mEq/kg/day 0.3-0.5mEq/kg over 3hours of 50% solution |
|
Elemental iron |
Oral |
5-6mg/kg/day |
|
Folic acid |
Oral |
0.2mg/kg/day |
Manage bile flow with pruritu: 4,8,10
Maintenance of bile flow along with management of pruritus much necessary to maintain quality of life as well as prevent any complication (Table 6). 2
|
Drugs |
Routes |
Dose |
|
Ursodeoxycholic acid |
Oral |
20-30mg/kg/day 3 divided doses |
|
Rifampicin |
Oral |
10mg/kg/day |
|
Phenobarbital |
Oral |
5-10mg/kg/day |
|
Cholestyramine |
Oral |
250mg/kg/day |
Neonatal phototherapy in cholestasis 2,10
Bronze baby syndrome is the dark gray-brown pigmentation of skin, mucous membrane and urine following phototherapy that occurs in some infants with cholestasis caused by a poorly understood accumulation of the bilirubin photoisomer, and/or porphyrins, and other metabolites, and/or biliverdin.2,10,37 This syndrome generally recovers spontaneously and cholestasis does not contraindicate phototherapy. The American Academy of Pediatrics (AAP) suggests that the direct (conjugated) serum bilirubin should not be subtracted from the total serum bilirubin concentration in making decisions about exchange transfusions.2,10,38 Moreover, it suggests that in infants who develop the bronze baby syndrome, exchange transfusion should be considered when the total serum bilirubin is in the intensive phototherapy range and phototherapy does not promptly lower the total serum bilirubin. In fact, it has been reported that bronze baby syndrome may be an additional risk of developing kernicterus.2,10,39 However, the paucity of data do not permit firm recommendations on this topic.
Specific medical or surgical intervention
It is crucial to rapidly identify infants who have medically treatable forms of cholestasis as well as those causes amenable to surgical intervention (Table 7).4,9,10
|
Causes of cholestasis |
Intervention |
|
BA |
Hepatoportoenterostomy (Kasai procedure) |
|
CC |
Corrective surgery |
|
INH |
MCT oil, UDCA, Phenobarbitone, Vit A,D,E,K, Zinc, Folic acid, Calcium |
|
|
|
|
Viral infections CMV HSV Toxoplasma |
Valganciclovir Acyclovir Salfadiazine, Pyrimethamine, Folinic acid |
|
Bacterial infection (UTI, Sepsis) |
Appropriate antibiotic |
|
Hypothyroidism |
Thyroid hormone replacement |
|
Hypopituitarism |
Thyroid, growth hormone, cortisol replacement |
|
Galactosemia |
Lifelong galactose-free diet |
|
Tyrosinemia |
Nitisinone (1 mg/kg/day) Low tyrosine/phenylalanine diet, Liver transplantation |
|
Hemochromatosis (Neonatal)
|
Blood exchange transfusion Intravenous Immunoglobulin therapy Liver transplantation |
|
PFIC |
Ileal exclusion, partial external biliary diversion, liver transplantation |
|
Alagille syndrome |
Liver transplantation |
|
A1AT deficiency Cystic fibrosis |
Liver transplantation Pancreatic enzymes, ursodeoxycholic acid |
|
BASD |
Cholic acid and its derivatives |
|
Inspissated bile in the common bile duct Hereditary fructose intolerance |
Biliary tract irrigation Fructose- and sucrose-free diet |
|
PLBW |
Enteral feeding, Fish-oil based lipid emulsions, UDCA |
Table 7 Causes of Cholestasis that require Specific Medical or Surgical intervention
Kasai’s PE consists of removal of the atretic extrahepatic tissue and a Roux-en-Y jejunal loop anastomosis to the hepatic hilum. PE may be considered successful if serum bilirubin normalizes after surgery. 4 The rate of success in re-establishing bile flow is dependent on the age of the infant when the hepatic PE is performed as well as on the experience of the surgeon (Figure 22).10 There is up to an 80 % success rate if the surgery takes place at less than 30 to 45 days of age; however, fewer than 20 % of patients who undergo hepatic PE at older than 90 days achieve bile drainage. 10,40,41,42
The success of surgery is shown by the excretion of bile and improvement of jaundice (Figure 23). The most significant predictive factor of long-term prognosis is resolution of jaundice. Patients who remain jaundiced usually die or have LT by 8years age. Jaundice-free patients have a 10-year survival of almost 90 %. However, the majority of patients with BA have progressive disease, with at least 80 % requiring LT by age 20years.10,43
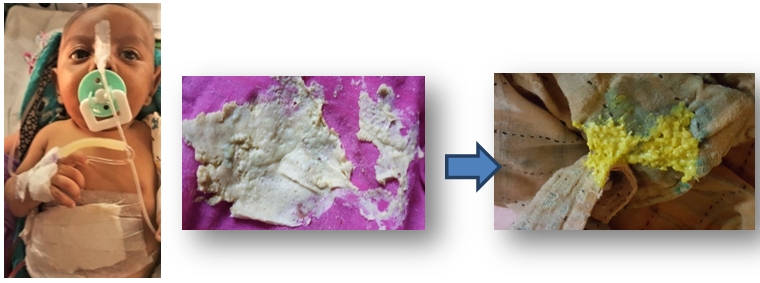
Figure 23: Stool color (before and after Kasai’s portoenterostomy)
(Picture was taken with permission).
Surgery gives excellent results for CC (Figure 24) and should be performed as soon as the diagnosis is made.
In children with PFIC without decompensated cirrhosis, external and internal biliary diversion has been shown to be of benefit. On the other side, with decompensated cirrhosis, LT is the answer. 44
Liver transplantation
Any baby, who has had Kasai’s PE and the bilirubin remains >6mg/dL, three months after surgery, should be referred to a transplant center. Babies with BA who present with decompensated cirrhosis (low albumin, prolonged INR, ascites) are not likely to improve with a Kasai PE and should be referred for LT (Figure 25). Of the 355 transplants in children that have been performed in India till 2012, 30% have been for BA. 4,45 Living related LT (the vast majority of liver transplants in India are living related), performed at experienced centers, is associated with favorable outcomes, with 5- and 10-year survival rates of 98% and 90%, respectively.4,46-48
Figure 25: Biliary atresia infant waiting for Liver Transplantation
(Picture was taken with permission)
Screening & prevention
BA is the most common cause of NC and progresses to end-stage liver disease in up to 80% of patients within the first two decades after birth. Early identification and Kasai PE are essential to establish bile flow and avoid LT within the first 2years. 2,40 A loss of stool pigmentation (acholic stools) may be one of the earliest clinical indicators of BA and is not confounded by breastfeeding, as is relying solely on the presence of jaundice. Lai et al 2,49 found that 95% of infants who have BA had acholic stool in early infancy. In Taiwan & Japan, a national stool color screening system was implemented and SCC was placed into the child health booklet given to the mother of every newborn.2,50,51 Mothers were to notify a care provider if the infant had an acholic stool before age 1month and brought the SCC into the 1-month health supervision visit to show the provider the color of the stools. This program reduced the average age at diagnosis of BA, increased the national rate of the PE operation performed before age 60 days, increased the 3-month jaundice-free rate after PE, and increased the 5-year overall survival rate. Currently, the AAP does recommend obtaining a total serum bilirubin or transcutaneous bilirubin level in all newborns before discharge from the hospital. 2
Cholestatic jaundice in an infant is a typical presenting feature of neonatal liver disease and is frequently clinically confused with the more common prolonged unconjugated hyperbilirubinemia. NC constitutes almost one-third of children with chronic liver disease. BA, neonatal hepatitis and metabolic causes are the most important etiology. Lots of treatable condition in your desk if recognize early. This requires specific biochemical tests, imaging studies and interpretation of histopathology by experienced personnel. Late diagnosis of BA must be avoided because its surgical treatment is much more successful when performed before 30 to 45days of life. The overall outcome in South-East Asia is far from satisfactory, due to late referral. The mean age at presentation in tertiary care centers is still over 3 months compared to recommended age of less than 60 days.
Careful history, thorough physical examination, and fractionation of serum bilirubin are recommended in any infant with jaundice seen after 2 weeks of life. SCC may added in the Expanded Program on Immunization (EPI) program for increase awareness of health workers, physicians and also parents. The screening program with these card could be a useful tool for avoiding the late referral as well as improving the prognosis of patients with BA in the next future. When NC leads to significant liver failure or decompensated cirrhosis, LT should be the option.
Not applicable.
SM was a major contributor to the manuscript writing, prepared the first draft of this paper and revising. MSS, AD and SSA collected, critically interprited the study data and contributed in the manuscript writing. All authors read and approved the final manuscript.
No fund was received for this article.
The authors declare that they have no competing interests.

©2021 Mahmud, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.