eISSN: 2373-6372


Review Article Volume 13 Issue 6
Department of Pharmacology, Medical Research and Clinical Studies Institute, Egypt
Correspondence: Manal ME Ahmed, Department of Pharmacology, Medical Research and Clinical Studies Institute, National Research Centre, Giza, Egypt, Tel +20-10936-27027
Received: September 12, 2022 | Published: November 4, 2022
Citation: Ahmed MME. MRSA infections: priorities and future approaches for research. Gastroenterol Hepatol Open Access. 2022;13(6):200-208. DOI: 10.15406/ghoa.2022.13.00523
Methicillin-resistant Staphylococcus aureus (MRSA) has arisen since the 1960s, spread around the world, and become a major cause of bacterial infections in both healthcare and community settings. However, the prevalence of MRSA varies significantly by region due to a number of reasons, including variations in local infection control procedures and pathogen-specific traits of the circulating clones. The independent acquisition of staphylococcal cassette chromosome mec (SCCmec), which contains genes encoding proteins that render the bacterium resistant to the majority of β-lactam antibiotics (such as methicillin), by several S. aureus clones, has led to the emergence of various MRSA clones. The abundance of virulence factors that S. aureus produces, along with β-lactam resistance and, for the majority of clones, resistance to other antibiotic classes, contribute to MRSA’s success. Clinical signs of MRSA can include asymptomatic nasal mucosa colonization, moderate skin and soft tissue infections, or fulminant invasive illness with a high fatality rate. Although there aren’t many choices for treating MRSA, numerous new antibiotics are in the works.
It will be possible to optimise strategies to effectively control MRSA if you have a thorough understanding of colonisation dynamics, transmission pathways, risk factors for progressing to infection, and environmental factors that encourage the evolution of resistance. Additionally, vaccine candidates are being developed and may eventually be useful as a preventative approach.
Keywords: MRSA, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, staphylococcal cassette chromosome mec, β-lactam
MRSA, methicillin-resistant Staphylococcus aureus; SCCmec, staphylococcal cassette chromosome mec, ClfB, clumping factor B; PMNs, polymorphonuclear leukocytes; PBP2a, penicillin-binding protein 2a; IgG, immunoglobulin G; CP5, capsular polysaccharide 5; WTA, wall teichoic acid; WGS, whole-genome sequencing
The Coccoid bacteria Staphylococcus aureus belongs to the Firmicutes phylum and is Gram-positive, nonmotile, and coagulase-positive. S. aureus is by far the most clinically relevant of the 52 species and 28 subspecies that make up the Staphylococcus genus (List of Prokaryotic names with Standing in Nomenclature). 20–40% of the general population has S. aureus in their nasal mucosal commensal microbiota.1,2 Due to variations in the size and demography of the study populations, the quality of the sample, and the culture techniques used,3 the stated prevalence varies. S. aureus can enter underlying tissues or the bloodstream and spread infection when the cutaneous and mucosal barriers are compromised, such as due to chronic skin conditions, wounds, or surgical intervention. S. Aureus infection is more likely to occur in people with invasive medical devices (like peripheral and central venous catheters) or weakened immune systems.4 The first case of methicillin-resistant S. aureus (MRSA) was reported in England in 1961,5 not long after the drug was first used in clinical settings. Methicillin was once widely used, but due to its toxicity, it is no longer marketed for human use. Instead, similar, more stable penicillins like oxacillin, flucloxacillin, and dicloxacillin6 have largely taken its place. However, the phrase “methicillin-resistant S. Aureus ” is still in use. MRSA was the cause of hospital epidemics (healthcare-associated MRSA, or HA-MRSA) in various regions of the world in the decade after its initial description.7 The occurrence of community-associated MRSA (CA-MRSA), also known as MRSA, marked a significant shift in the epidemiology of the bacteria. It has also been linked to exposure to livestock since the middle of the 2000s (livestock-associated MRSA, or LA-MRSA).8 Several S. aureus clones have evolved into MRSA through horizontal gene transfer of staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that encodes the genes mecA and mecB. Clones are bacteria that cannot be distinguished from one another by a variety of genetic tests (for example, pulsed-field gel electrophoresis, multilocus enzyme electrophoresis, or ribotyping), or that are so similar that they.9,10 MRSA frequently exhibits resistance to antibiotics with β-lactams and numerous other antibiotic groups. The remarkable capacity of S. aureus to develop resistance to any antibiotic7 has significant ramifications for both existing and potential future treatments for this pathogen.
People who have MRSA colonisation or carriage—i.e., the presence of bacteria that do not result in cellular damage, clinical signs and symptoms of infection, or detectable host immune responses—have a higher risk of contracting the infection again and are a significant source of person-to-person transmission. People who are susceptible to illness are housed in healthcare institutions, settings with strong antibiotic selection pressure (which might lead to the selection of antimicrobial resistance in bacteria, for instance, because to invasive procedures and/or immunological impairment) and frequent human contact. MRSA is now endemic in many healthcare facilities around the world, and as a result, it has become a major focus for infection control efforts globally. These conditions have facilitated the epidemic spread of MRSA in hospitals.
Future research priorities are covered in this primer, which also addresses the mechanisms, pathophysiology, prevention and management of MRSA (Figure 1).

Figure 1 Most frequent MRSA clones. Sequence types (STs) of methicillin-sensitive Staphylococcus aureus (MSSA) are grouped into clonal complexes (CCs) by their similarity to a founder allelic profile (genotype)226. STs have different molecular properties that enable monitoring of the geographical spread of different clones. STs of MSSA can evolve into MRSA by acquiring staphylococcal cassette chromosome mec (SCCmec), of which there are different types (represented by roman numerals). Commonly used clone names are within parentheses. Numbers in the names of MRSA USA clones are ased on pulsed- field gel electrophoresis analysis.
ACME, arginine catabolic mobile element; EMRSA, epidemic MRSA; PVL, Panton–Valentine leukocidin; sek2 and seq2 are staphylococcal genes encoding enterotoxin.80
Mechanisms/pathophysiology
Colonisation by Staphylococcus aureus
Most of the time, S. aureus colonisation occurs before an infection occurs.11 Less frequently, infection can happen when S. aureus colonisation is unknown, for instance, when catheters or wounds are contaminated due to subpar infection control procedures by healthcare professionals. Although colonisation occurs at other sites, most notably in the throat and perineum,12 the nose is the primary site of S. aureus colonization. Three temporal patterns of S. aureus (including MSSA an MRSA) colonisation have been identified by longitudinal studies.13 Only 15% of people were found to have continuous S. aureus colonisation (known as persistent carriers), while 70% of people had intermittent colonization, which indicates that most people can repeatedly acquire S. aureus and spontaneously clear it., and 15% of people (known as non-carriers) had no evidence of S. aureus.13 Other studies14 found comparable outcomes. There are underlying host factors that determine the carriage status, according to studies examining particular host polymorphisms in genes related to the inflammatory response. These studies, which examined these genes,15 However, it is not quite clear what these underlying variables exactly are. The length of colonisation can vary, especially for MRSA, and quoted estimates may be skewed by antibiotic use, which can cut the length of colonisation. The median time of colonisation in one research of patients with MRSA colonisation at the time of hospital release was 282 days,16 and 81% of those in this sample had persistent skin lesions, which is a known risk factor for MRSA colonisation.that might have caused the protracted carriage. Along with host variables, the nasal microbiota and factors related to the virus itself might affect the host carrier status.
Colonisation dynamics
Teichoic acid on the cell wall serves as a mediator during the early stages of S. aureus colonisation while microbial surface components that recognise adhesive matrix molecules are involved during the later stages of nasal colonization.17,18
S. aureus clumping factor B (ClfB), one of these components, has been researched in vitro and on human volunteers.19 The nose was injected with both the wild-type strain and its single locus clfB knockout variation; the knockout version cleared much more quickly than the wild-type strain.ClfB-deficient strains, however, may still interact with nasal cells, suggesting that there are a number of distinct microbial surface elements that contribute to colonization.20 It should be noted that this study only used one strain.
Along with host and pathogen factors, S. aureus interacts with other species that colonise the nose, such as Corynebacterium spp., Propionibacterium acnes, Staphylococcus lugdunensis, and Staphylococcus epidermidis. According to research on the nasal microbiota, certain species (such as S. Epidermidis, which has been positively correlated with S. aureus presence) are associated with the presence or absence of S. aureus.21,22 The organisms that make up the nasal microbiota compete with one another in a number of ways. For instance, they struggle for adhesion sites and nutrition because the human nose has few of both.
S. aureus is more adapted to the human nose because it can survive in environments with nutrient levels that are lower than coagulase-negative staphylococci can23 (possibly due to differences in metabolism). However, there hasn’t been any evidence of a difference in nutritional levels between carriers and non-carriers.23 Species of the microbiota can also engage in antibiosis, or the production of chemicals that prevent their competitors from growing. For instance, S. Lugdunensis creates lugdunin, an antimicrobial compound that inhibits and kills S. aureus (including MRSA) in a mouse model and in vitro, possibly by causing a rapid breakdown of bacterial energy resources.24 A sixfold lower risk of nasal colonisation with S. aureus has been linked to nasal colonisation with S. lugdunensis in humans. Although these findings are intriguing, they only partially account for carriage patterns since S. lugdunensis colonisation has only been documented in 9–26% of the general population.24,25 Last but not least, S. aureus also engages in competitive host defences, causing the host to produce antimicrobial proteins that are less toxic to S. aureus than to other commensal bacteria.26 Numerous studies back up the involvement of these mechanisms in the interactions between S. aureus and the commensal microbiota, but only one mechanism can fully account for all carriage patterns that have been seen (Figure 2).
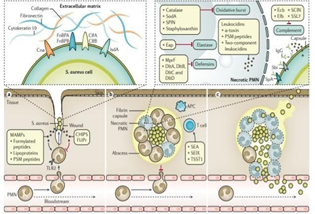
Figure 2 Stages of Staphylococcus aureus infection. A | Bacteria obtain access to sterile tissues via open wounds and use adhesin proteins, such as fibronectin-binding protein A (FnBPA), FnBPB, iron-regulated surface determinant protein A (IsdA), clumping factor A (ClfA), ClfB and collagen adhesin (Cna), for specific attachment to extracellular matrix proteins, such as fibronectin, cytokeratin and collagen, among others. Staphylococcus aureus can also in part regulate polymorphonuclear leukocyte (PMN) influx in subtle ways involving activators (formylated peptides and phenol-soluble modulin (PSM) peptides) and inhibitors (for example, chemotaxis inhibitory protein of S. Aureus (CHIPS) and FPRL1 inhibitory protein (FLIPr)) of PMN chemotaxis. PSM peptides also promote the release of pro-inflammatory lipoproteins, the major S. Aureus microorganism-associated molecular pattern (MAMP) molecules, which activate Toll-like receptor 2 (TLR2) and contribute to local inflammation. B | S. Aureus produces coagulases to polymerize fibrin and form an encapsulated abscess around the infection site. The capacity of PMNs, which are found in high numbers in an abscess, to eliminate S. Aureus is limited by leukocidins and by virulence factors interfering with opsonophagocytosis and PMN killing. S. Aureus can compromise effective opsonization by antibodies using a polysaccharide microcapsule and surface proteins (Staphylococcus protein A (SpA) and immunoglobulin-binding protein Sbi) binding immunoglobulin G (IgG) via the crystallizable fragment (Fc) domain in a futile way66. The bacteria can also inhibit the complement signalling pathway by small secreted inhibitors such as staphylococcal complement inhibitor (SCIN), fibrinogenbinding protein (Efb), extracellular complement- binding protein (Ecb) or staphylococcal superantigen-like protein 7 (SSL7), among others. Phagocytosed bacteria can survive within the PMNs by producing catalase, superoxide dismutase [Mn] 1 (SodA), staphylococcal peroxidase inhibitor (SPIN),staphyloxanthin (against the bactericidal oxidative burst generated by the PMNs)72 and extracellular adherence protein (Eap) (against elastase), and the cell envelope modifications mediated by multiple peptide resistance factor (MprF) and the d-alanine transfer proteins DltA, DltB, DltC and DltD protect against defensins. S. Aureus also secretes cytolytic toxins that can kill PMNs; S. aureus leukocidins include large pore-forming proteins (α-toxin and several two-component leukocidins, such as Panton–Valentine-Leukocidin (PVL)) and small peptide (PSM peptides) toxins. Superantigen toxins (toxic shock syndrome toxin 1 (TSST1), enterotoxin type A (SEA), staphylococcal enterotoxin-like X (SEIX) and several others) contribute to exuberant inflammation by nonspecific T cell activation. C | Abscesses can release live bacteria to the surface of the skin and/or the bloodstream at later stages; the plasminogen-activating protein staphylokinase might contribute to bacterial dissemination. APC, antigen-presenting cell.81
Virulence
The wide range of infections that S. aureus can cause27,28 is a reflection of its extensive arsenal of virulence factors, which include adhesive, host-cell damaging, and immuno-modulatory molecules and can vary in their presence or specificity between clones.29,30 Since a large number of virulence genes are present on mobile genetic elements, their combination varies greatly between clones and even between strains that are distantly related. Because many of these virulence factors have redundant or partially overlapping functions, it is still unclear whether they are associated with particular types or levels of aggressiveness in S. aureus infections. Additionally, because they are human-specific, many virulence variables cannot be studied in animal models.31 The most notable virulence mechanisms and common entry points are the main topics of this section (Table 1).
Type of virulence factors |
Virulence factors |
Corresponding host ligands |
MAMPs |
||
Chemotactic MAMPs |
*Formylated peptides |
* N-Formyl-peptide receptor (FPR) 1 and FPR2 |
*Phenol-soluble modulins (PSMs) |
||
Non-chemotactic MAMPs |
* Lipoproteinsb |
* Toll-like receptor (TLR) 2 and TLR9 |
* DNA |
* Nucleotide-binding oligomerization |
|
* Peptidoglycan |
domain-containing protein 2 (NOD2) |
|
Adhesins |
||
Surface proteins |
* Fibronectin-binding protein A (FnBPA) and FnBPB |
* Fibronectin |
* Collagen adhesin (Cna)b |
* Collagen |
|
* Iron-regulated surface determinant protein A (IsdA) |
* Cytokeratin 10 (also known as KRT10) |
|
* Loricrin |
||
Glycopolymers |
* Wall teichoic acid (WTA) |
Scavenger receptors |
Evasins |
||
MAMP receptor inhibitors |
* Chemotaxis inhibitory protein of S. aureus (CHIPS)b |
FPR1 |
* FPR-like 1 (FPRL1) inhibitory protein (FLIPr)b |
•C5a anaphylatoxin chemotactic receptor (C5aR1) |
|
* FLIPr-likeb |
• FPR2 |
|
* Staphylococcal superantigen-like protein 3 (SSL3)b |
• TLR2 |
|
* SSL5b |
||
Chemokine receptor inhibitors |
* SSL5b |
* Several chemokine receptors |
* SSL10b |
||
PMN extravasation inhibitors |
* SSL5b |
* P-Selectin glycoprotein ligand 1 (PSGL1) |
* Extracellular adherence protein (Eap)b |
* Intercellular adhesion molecule 1(ICAM1) |
|
Coagulation factors |
* Coagulase (Coa) |
* Prothrombin |
* Secreted von Willebrand factor binding protein (vWbp) |
* Fibrinogen |
|
* Clumping factor A (ClfA) |
||
* ClfB |
||
Anticoagulants |
* Staphylokinaseb |
* Plasmin |
Complement inhibitors |
* Zinc metalloproteinase aureolysin |
* Complement proteins C3, C3b, C3bBb and C5a |
* Staphylococcal complement inhibitor (SCIN)b |
* Complement factor H |
|
* Fibrinogen-binding protein (Efb)b |
||
* Extracellular complement-binding protein (Ecb, also known |
||
as extracellular fibrinogen-binding protein)b |
||
* SSL7b |
||
* Immunoglobulin-binding protein Sbib |
||
Opsonophagocytosis inhibitors |
* Staphylococcus protein A (SpA) |
* Immunoglobulin G (IgG) |
* Immunoglobulin-binding protein Sbib |
* Immunoglobulin-γ receptor (FcγR) |
|
* Microcapsuleb |
||
* FLIPrb |
||
Synthases of anti-phagocytic mediators |
* Adenosine synthase (AdsA) |
* Adenosine monophosphate |
Inhibitors of PMN killing |
* Catalase |
* Reactive oxygen species |
* Superoxide dismutase [Mn] 1 (SodA) |
* Elastase |
|
* Staphyloxanthin |
* Lysozyme |
|
* Eapb |
* Defensins |
|
* Staphylococcal peroxidase inhibitor (SPIN) |
* Neutrophil extracellular traps (NETs) |
|
* O-Acetyltransferase A (OatA) |
||
* Multiple peptide resistance factor (MprF) |
||
* d-alanine transfer protein A (DltA), DltB, DltC and DltD |
||
* Thermonuclease (Nuc) |
||
Toxins |
||
Pore-forming protein toxins |
* α-Toxin |
* Disintegrin and metalloproteinase |
* Bi-component γ-Haemolysin (Hlg) AB |
domain-containing protein 10 (ADAM10) |
|
* Bi-component HlgCB |
* Several chemokine receptors |
|
* Leukocidin (Luc) EDb |
* Duffy antigen/chemokine receptor (DARC, |
|
* LucAB |
also known as atypical chemokine receptor 1) |
|
* Panton–Valentine leukocidin (PVL)b |
* C5aR1 |
|
Pore-forming peptide toxins |
* PSMα1–PSMα4 |
* Host cell membranes |
* PSMβ1 and PSMβ2 |
||
* SCCmec-encoded PSM (PSMmec)b |
||
* δ-Toxin (also known as δ-haemolysin, Hld) |
||
Toxins (cont.) |
||
Superantigen toxins |
* Toxic shock syndrome toxin 1 (TSST1)b |
* Major histocompatibility complex (MHC) class II |
* Enterotoxins types (SE) A-Qb |
* T cell receptor |
|
* Staphylococcal enterotoxin-like X (SEIX)b |
||
Sphingomyelinase |
* β-Haemolysin (Hlb)b |
* Sphingomyelin |
Proteolytic toxins |
* Exfoliative toxins (Etx)b |
* Desmoglein 1 |
Table 1 Major Staph. aureus virulence factors and corresponding host ligands83
Starting of infection
S. aureus SSTIs are typically started by bacterial transfer from the main reservoir in the nose to open micro-lesions and wounds on the skin.32,33 This transfer is most likely caused by hand contact. S. aureus surface proteins that bind to extracellular matrix proteins, such as fibronectin-binding protein A (FnBPA), FnBPB, clumping factor A (ClfA), ClfB, and collagen adhesin (Cna), allow the bacteria to adhere to and grow on damaged tissues.34 S. aureus is a common cause of catheter-associated infections, joint replacement infections, and ventilator-associated pneumonia due to its ability to adhere to and form biofilms (sticky agglomerations of microorganisms embedded in an extracellular matrix that facilitate resistance to mechanical interference, host defences, and antibiotic treatment) on artificial plastic or metal surfaces.35 S. aureus manipulates the subsequent influx of polymorphonuclear leukocytes (PMNs),36 which shapes local inflammation.37
Abscess formation
Infiltrating PMNs and bacteria are surrounded by a fibrin pseudo-capsule that formed by the S. aureus coagulase proteins, which inhibits further leukocyte influx.38 For example, S. aureus can prevent opsonization by producing a polysaccharide microcapsule and blocking the complement cascade.39 However, crucial MRSA clones like USA300 lack the microcapsule (REF.63). In addition to resisting PMN death mechanisms.40,41 bacteria that are phagocytosed by PMNs can also survive killing over time with the use of cytolytic toxins. For instance, many CA-MRSA clones produce α-haemolysin, pore-forming peptides (phenol soluble modulins, or PSMs) and a number of bi-component leukocidins, such the Panton-Valentine leukocidin (PVL), that are host species-specific and bind to host leukocyte membranes, causing holes to develop and lytic cell death to occur.42 so boosting bacterial virulence. S. aureus superantigen toxins, which bind to MHC class II antigen-presenting cells and activate a significant portion of T cells non-specifically, exacerbate the severe inflammation induced by activated or necrotic PMNs. This systemic hyper-inflammation is known as “cytokine storms.”43
Fulminant systemic inflammation
At a later stage, abscesses may rupture, releasing pus and live bacteria either onto the skin’s surface to aid in the spread of pathogens or into the bloodstream to result in bacteremia. Endovascular S. aureus has the ability to stick to endothelial surfaces and platelets.44,45 This adhesion can cause endocarditis, encourage the growth of metastatic abscesses, or cause bacterial uptake into endothelial cells, where the bacteria are difficult for antibiotics and host defence molecules to reach.46 Systemic blood coagulation is thought to be aided by coagulases’ agglutinating activity, and if the endovascular spread of the bacteria is not controlled, massive releases of cytokines and molecules associated with microorganisms result in sepsis, multi-organ failure, and fulminant systemic inflammation.47
Adaptation and regulation
The quorum-sensing system of the accessory gene regulator (Agr) and other regulatory networks48 are responsible for differentially regulating the majority of S. aureus virulence factors. Many CA-MRSA clones, including USA300, have extremely active Agr systems, which promote the profuse expression of toxins and are associated with a high propensity to infect even healthy people with SSTIs and invasive infections.49 In contrast, many HA-MRSA clones also have a phenol-soluble modulin that is encoded by the SCCmec (PSM; PSMmec), whose mRNA inhibits the expression of Agr.50 As a result, Agr is not highly active in many HA- MRSA clones, which produce more adhesins than toxins and frequently lead to bacteraemia through contaminated catheters or implanted medical devices. High virulence may even be harmful for S. aureus in bacteremia, as evidenced by the discovery of many isolates from bloodstream infections carrying point mutations that render Agr inactive.51 For the development of novel preventive and therapeutic approaches against MRSA, it will be essential to elucidate the virulence mechanisms whose inhibition would make S. aureus most vulnerable.
Methicillin resistance mechanisms
The independent acquisition of the SCCmec complex by several multidrug-resistant strains (resistant to penicillin, streptomycin, tetracycline, and erythromycin) in the early 1960s, which made S. aureus resistant to the majority of members of the β- lactam family of antibiotics,52 was a significant event in the evolution of S. aureus. The cassette chromosome recombinase (ccr) complex type and the class of the mec complex are used to categorise the twelve known SCCmec kinds (I-XII) (Table 2). Large SCCmec elements of Types I, II, and III, which are typically seen in HA- MRSA,53 include genes that confer resistance to various antibiotic classes. CA- MRSA, such as USA300 and USA400, contains smaller elements, such as kinds IV and V SCCmec, as well as in a few well-known HA-MRSA clones, as ST22-MRSA-IV, ST45-MRSA-IV, and ST5-MRSA-VI. The line separating the two epidemiological groups (HA-MRSA and CA-MRSA) has, however, blurred over time (Table 2).54
SCCmec types |
mec determinant |
ccr gene complexesa |
mec gene complexes |
High-prevalence setting |
I |
mecA |
1 (A1B1) |
B |
HA-MRSA |
II |
mecA |
2 (A2B2) |
A |
HA-MRSA |
III |
mecA |
3 (A3B3) |
A |
HA-MRSA |
IV |
mecA |
2 (A2B2) |
B |
CA-MRSA and HA-MRSA |
V |
mecA |
5 (C1) |
C2 |
CA-MRSA and HA-MRSA |
VI |
mecA |
4 (A4B4) |
B |
HA-MRSA |
VII |
mecA |
5 (C1) |
C1 |
NAb |
VIII |
mecA |
4 (A4B4) |
A |
NAb |
IX |
mecA |
1 (A1B1) |
C2 |
NAb |
X |
mecA |
7 (A1B6) |
C1 |
NAb |
XI |
mecC |
8 (A1B3) |
E |
LA-MRSA |
XII |
mecA |
9 (C2) |
C2 |
NAb |
Table 2 Currently identified SCCmec types in Staphylococcus aureus strains
(Parentheses indicate the ccr gene(s) in the ccr gene complex. B Not possible to assign this SCCmec type because there is insufficient information concerning its occurrence. CA-MRSA, Community-associated methicillin-resistant Staphylococcus aureus; HA-MRSA, health-care-associated methicillin-resistant Staphylococcus aureus; LA-MRSA, livestock-associated Methicillin-resistant Staphylococcus aureus; NA, not applicable. Adapted with permission From International Working Group on the Staphylococcal Cassette Chromosome elements).
All SCCmec types have mecA, which encodes penicillin-binding protein 2a (PBP2a), a peptidoglycan transpeptidase, with the exception of type XI, which contains the homologue mecC.55 PBP2a can take over the transpeptidase function of peptidoglycan biosynthesis in the presence of β-lactam antibiotics that inhibit the activity of the four native S. aureus penicillin-binding proteins (PBP1, PBP2, PPB3, and PBP4). PBP2a has a very low affinity for most β -lactam antibiotics. MecA encodes PBP2aLGA, which is named after the MRSA strain LGA251 from which it was first isolated. MecC, a variant of mecA, was discovered in several S. aureus clones from animal and human isolates.56 When compared to MRSA strains that carry the mecA gene, the LGA251 strain’s control of β-lactam resistance has a different mechanism.57,58 The level of methicillin resistance in the LGA251 strain is dependent on the mecC gene as well as genes in the strain’s genetic background.
S. aureus has been found to have plasmid-borne methicillin resistance based on mecB in 2018, but the mechanism of resistance encoded by mecB has not yet been elucidated.59 MecA is primarily regulated by the regulators encoded by the genes mecI, mecR1 and mecR2,60,61 as well as by the regulators of the expression of the genes blaZ, blaI, and blaRI.62 A surprising number of auxiliary or accessory genes also have a significant impact on the resistance phenotype.63 The degree of methicillin resistance cannot be predicted by the level of mecA transcription, according to three lines of data. First, the antibiotic mupirocin induces a stringent stress response, or the bacterial response to various stress conditions such as amino acid, fatty acid, and iron limitation as well as heat shock, which results in an increase in PBP2a activity without affecting mecA transcription.64 Second, inactivating vraS (a component of the two-component regulatory system including sensor protein VraS and response regulator protein VraR (VraS-VraR) involved in the control of the cell wall peptidoglycan biosynthesis) increased mecA transcription but not PBP2a activation.65 Thirdly, PrsA, a chaperone foldase protein, modifies the amount of correctly folded PBP2a in the membrane and, consequently, methicillin resistance without influencing the transcription of mecA.66 Utilizing various experimental techniques, it has been demonstrated that the severe stress response is essential for mecA expression.67 Finding inhibitors of the stringent stress response that interact with -lactam antibiotics is the subject of a new line of research.67 Notably, since the 1960s, vancomycin, the first-line treatment for invasive MRSA infections in hospitalised patients, has developed resistance in several MRSA clones (Figure 3).68
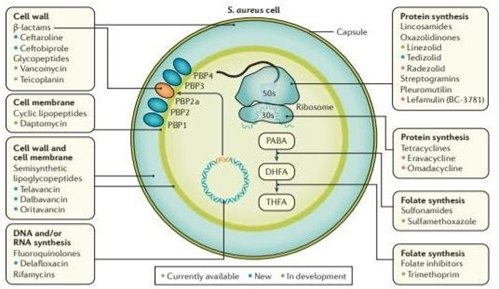
Figure 3 Bacterial targets of antibiotics active against MRSA. Antibiotics have diverse mechanisms of action and target different bacterial structures or metabolic pathways. Existing antibiotic options are in green, new antibiotics approved and on the market are in blue and antibiotics in the pipeline are in orange.
DHFA, dihydrofolic acid; PABA, para-aminobenzoic acid; PBP, penicillin-binding protein; S. Aureus, Staphylococcus aureus; THFA, tetrahydrofolic acid.82
Vaccines
The incidence and course of MRSA infections could be significantly impacted by developing vaccines. Infections do occur more frequently in S. aureus carriers, but they are also less severe69 than infections that develop in non-carriers, suggesting that sustained exposure to S. aureus antigens can result in protective immunity. A vaccine would ideally also hinder S. aureus colonisation in order to prevent infections from occurring in the first place, greatly reducing the need for antibiotic treatment and intensive infection control measures.70 Therapeutic monoclonal antibodies, such as the passive vaccination produced by Medimmune (Gaithersburg, Maryland, USA) against α-haemolysin, may open up new therapeutic avenues, either by themselves or in conjunction with antibiotics. The next few years won’t see the development of a S. aureus protective vaccine, despite intensive research and development efforts. In early clinical testing, two monovalent vaccine candidates were unsuccessful in producing sufficient protection. Both the StaphVax (Nabi Biopharmaceuticals, Rockville, Maryland, USA) and the V710 (Merck, Kenilworth, New Jersey, USA) vaccines, which contain the capsular polysaccharide 5 (CP5) and CP8 antigens, have been shown to be protective in animal models but not in placebo-controlled phase III trials.71,72 The lack of CP expression in several significant S. aureus clones, such as the significant MRSA clone USA300, the absence of adjuvants in the antigen preparations, and inconsistent immune responses to the used antigens may all have contributed to the failure. There is also widespread concern that the many immune evasion mechanisms used by S. aureus, including immunoglobulin G (IgG)-binding protein A, may reduce the effectiveness of antibodies, that opsonizing antibodies may not be sufficient to promote protection and that toxin-neutralizing antibodies may be just as important as or even more than previously believed.73
Basic scientific developments offer hints for new, perhaps more effective immunisation strategies.The cell wall glycopolymer wall teichoic acid (WTA) has been identified as a dominant surface antigen,74 and immunoproteomics studies have assisted in elucidating the most immunogenic and protective S. aureus antigens.75 There have been a number of novel toxins discovered, whose neutralisation by antibodies may help provide protection. It is also now more obvious which T cell subsets are necessary for anti-S. immunity.76
Pharmaceutical companies are still working on polyvalent anti-S. aureus vaccines based on the bacteria’s surface proteins (ClfA) and polysaccharides (CP5 and CP8), secreted toxins (-toxin, LukS-PV, ESAT-6 secretion system extracellular protein A (EsxA) and EsxB), and membrane-bound lipoproteins that are involved in nutrient uptake (manganese transport system membrane). In preclinical infection studies, a novel WTA-targeting monoclonal antibody coupled to a rifampicin-related antibiotic shown protection.77 There is optimism that certain current vaccine development initiatives may result in the successful conclusion of clinical trials.
Priorities and new approaches for research:
MRSA and humanity will probably always cohabit. The biomedical research community would be well advised to continue its varied activities in the field of MRSA research notwithstanding the present emphasis on multidrug-resistant Gram-negative bacteria and the fall of HA-MRSA infections in some areas. MRSA continues to be a high-priority multidrug-resistant bacterium that requires continued efforts for the research and development of new antibiotics and cutting- edge prevention measures, as noted by a 2017 WHO report.78 In addition to vaccinations, bacteriophages or lytic proteins produced from bacteriophages may be employed for novel preventive measures, such as nasal MRSA decontamination in a time of rising mupirocin resistance.79 Overall, there are still many unanswered questions and significant problems to solve, which necessitate continued attention from academics, decision-makers in government policies, funders, and those in charge of MRSA treatment and management. In the 60 years after it was originally identified, MRSA has proven to have a remarkable capacity for change and widespread dissemination. Control of this extremely successful pathogen will ultimately be made easier by a number of factors, including improved understanding of the pathogenesis of infection, accurate and speedy diagnostics, assurance of the availability of effective treatment options, and optimization of the prevention of transmission and infection.
The future research needs for MRSA can be summarized in the following points:
None.
Author declares there are no confits of interest.
None.

©2022 Ahmed. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.