eISSN: 2373-6372


Research Article Volume 14 Issue 5
1Assistant Professor of Internal Medicine, Hepatology and Gastroenterology Unit, Internal Medicine Department, Specialized Medical Hospital, Faculty of Medicine, Mansoura University, Egypt
2Specialist of clinical pathology, Blood Bank Department, Mansoura New General Hospital, Ministry of Health, Mansoura, Egypt
3Lecturer of diagnostic radiology, Radiology Department, Mansoura University Hospital, Faculty of Medicine, Mansoura University, Egypt
4Assistant Professor of Internal Medicine, Faculty of Medicine, Cairo University, Egypt
Correspondence: Hany Shabana, Assistant Professor of Internal Medicine, Hepatology and Gastroenterology Unit, Specialized Medical Hospital, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Received: October 31, 2023 | Published: November 28, 2023
Citation: Shabana H, Askar M, Mohsen M. Mean platelet volume and platelet distribution width as predicting biomarkers of hepatitis C related hepatocellular carcinoma. Gastroenterol Hepatol Open Access. 2023;14(5):165-173. DOI: 10.15406/ghoa.2023.14.00563
Background and Aims: Besides their role in hemostasis and thrombosis; platelets have roles in innate and adaptive immune responses, atherosclerosis, lymphatic vessel development, angiogenesis and carcinogenesis .The relation between platelets and cancer is well-known and thrombocytosis may occur as a paraneoplastic syndrome. Several studies reported a correlation of high platelet count with poor prognosis in different malignancies. The aim of this study was to explore the predictive values of platelet count (PLC), mean platelet volume (MPV) & platelet distribution width (PDW) in HCV related HCC, as well as their association with tumor burden.
Method: The study was conducted on two groups of patients, group 1; included patients with naive HCV related HCC and group 2; included patients with chronic HCV cirrhosis. Liver biochemical tests, AFP, serum creatinine, multi-phasic contrast enhanced abdominal CT and or MRI were done at baseline for all cases. In Group 1, non-invasive diagnosis of HCC in cirrhotic patients according to the EASL guidelines & Barcelona Clinic Liver Cancer (BCLC) staging was done. In Group 2, liver cirrhosis was diagnosed through liver histology or a combination of radiographic, clinical, and laboratory findings. CBC was done for all participating patients using automated hematology analyzer with reporting of PLC, MPV& PDW.
Results: Group 1 included 144 patients, while group 2 included 116 patients. In group 1, the age was significantly older & the number of males was significantly higher. PLC was significantly lower in group 1 than group 2. In group 1, PLC was < 150× 109/L (thrombocytopenia) in 71.6% of the cases, while it was normal in 28.4% of them. The median HCC diameter was significantly larger in patients with normal PLC than thrombocytopenic patients. There was significant positive correlation of PLC with BCLC stage. PLC was significantly higher in late than early BCLC stage. In group 1; PLC at cut off value of 134.5×109/L predicted late BCLC stages; with sensitivity of 44.1%, specificity of 82.5%, AUC of 0.65 & p = 0.006. There was insignificant difference between both groups regarding PDW. There was insignificant difference between both groups regarding PDW. There was insignificant difference between both groups regarding the MPV. In group 1; PDW was ≥14.5% in 79% & <14.5% in 21% of the patients while MPV was ≥10.4 fl in 52.1% of the patients & <10.4 fl in 47.9% of them. There was insignificant correlation of MPV & PDW with BCLC stage. PDW at cut off value of 15.05% predicted HCV related HCC with sensitivity = 62, 2%, specificity = 58, 1 %, AUC = 57, 8 %, p = 0.043. There was significant negative correlation of the MPV with serum AFP level. MPV was significantly smaller in late than early BCLC stage. On multivariate logistic regression analysis, gender, patient status of performance (PS), Alpha fetoprotein (AFP), PLC, Albumin -Bilirubin (ALBI) grade and Model of End stage Liver Disease (MELD) score, were significant predictors of HCV related HCC, while age, PDW, MPV and Child -Turcotte -Pugh (CTP) score were not significant predictors.
Conclusion: PLC was significant predictors of HCV related HCC. HCV related HCC patients with normal PLC had larger tumor diameter. PLC positively correlated with BCLC stage. In HCV related HCC cases, PLC at cut off value of 134.5×109/L may predict late BCLC stages. PDW at cut off 15.05% may predict HCV related HCC. MPV was significantly smaller in late than early BCLC stages & it negatively correlated with serum AFP level. MPV was not significant predictors of HCV related HCC.
Keywords: platelet count, platelet distribution width, mean platelet volume, platelet indices, hepatocellular carcinoma, chronic hepatitis C, alpha fetoprotein, Barcelona clinic liver cancer staging, Mansoura, Egypt
AFP, Alpha fetoprotein; ALBI, albumin bilirubin; ALT, alanine amino transferase; ANOVA, analysis of variance; AST, aspartate amino transferase; AUC, area under the curve; BCLC, barcelona clinic liver cancer; CBC, complete blood count; CI, confidence interval; CRP, C-reactive protein; CT, computed tomography; CTP, child-turcotte-pugh; DAA, direct acting antivirals; DM, diabetes mellitus; EASL, European association for the study of liver; ECOG, Eastern Co-operative oncology group; ELISA, enzyme-linked immuno sorbent assay; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalized ratio; aMAP, age, male, albumin-bilirubin, platelets; MDCT, multi detector computed tomography; MELD, model of end stage liver disease; MPV, mean platelet volume; MRI, magnetic resonance imaging; mTOR, mammalian target of rapamycin; NPV, negative predictive value; OR, odds ratio; OS, overall survival; PLT, platelet; PLC, platelet count; PDW, platelet distribution width; PI, platelet indices; PPV, positive predictive value; PST, patient status of performance; PT, prothrombin time; PVT, portal vein thrombosis; RBCs, red blood cells; RA, rheumatoid arthritis; ROC, receiver operating characteristic curve; SD, standard deviation; SE, standard error; SLE, systemic lupus erythematosus; SMH, specialized medical hospital; Sn, sensitivity; Sp, specificity; THPO, thrombopoietin; US, ultra sound
Platelets are a nucleate, disc-shaped cells present in the blood with critical role in hemostasis and thrombosis.1,2,3 They also have roles in innate and adaptive immune responses, atherosclerosis, lymphatic vessel development, angiogenesis, and carcinogenesis.4–7 The relation between platelets and cancer is well-known and thrombocytosis may occur as a paraneoplastic syndrome.8–10 Several studies reported a correlation of high platelet count with poor prognosis in different malignancies.11–13 The interplay between tumor cells and circulating platelets is characterized by a reciprocal mechanism in which the tumor activates platelets, while platelets enhance tumor progression and metastasis.14 Hepatocellular carcinoma (HCC) usually develops in patients with liver cirrhosis.15,16 It represents the second leading cause of cancer related death in men and the sixth leading cause of cancer-related death in women, respectively.11 It is estimated that only 40% of HCCs are diagnosed at early stages.17 There is a specific connection between platelets and HCC. Thrombocytopenia has been associated with HCC development in cirrhotic patients with chronic hepatitis C (HCV) infection.18,19 However, it is unclear if thrombocytopenia predisposes chronic HCV patients to HCC development or is just a marker of advanced liver disease.20 Mean platelet volume (MPV) is the average peripheral blood platelet size. A lower MPV was found to be an independent predictor of a reduced OS in patients with various cancers.21–23 Platelet Distribution Width (PDW) reflects platelet anisocytosis due to the change of their size and shape; denoting platelet activation. A strong relationship between PDW and the patients’ prognoses in several types of cancer was reported.24–26 However, currently there is limited knowledge on the association of these platelet indices (PIs) with tumor biology, as well as their predictive role in patients with chronic HCV related HCC.
Aim of the work
The purpose of this study was to explore the predictive role of platelet count (PLC), platelet distribution width (PDW) & mean platelet volume (MPV) in HCV related HCC as well as the association of these PIs with the tumor burden.
This was a single centre study which included 144 naive HCV related HCC adult patients (group1) &116 HCV related cirrhotic adult patients (group 2) who attended the early detection of HCC clinic at Specialized Medical Hospital (SMH), Mansoura University, Egypt. Patients with history of other malignancies (3 cases in group 2); patients with history of autoimmune disorders such as systemic lupus erythematosus (SLE) (2 cases in group 2); rheumatoid arthritis (RA) (3 cases in group 2); type 1 diabetes mellitus (DM) (3 cases in group 2); patients who had positive ELISA test for both HCV& HBV (2 cases in group1, 3 cases in group 2); patients on anti-platelet drugs (5 patients in group 2); patients who had undergone splenectomy (3 cases in group 1& 2 cases in group 2); patients who received HCC related treatments (150 cases in group 1), were exclude (Figure 1).
The study duration extended from August 2022 to July 2023. All enrolled patients were subjected to detailed medical history, clinical evaluation, assessment of performance status (PST) by scale of the Eastern Cooperative Oncology Group (ECOG) (Table 1).
Grade |
ECOG performance status |
0 |
Fully active, the same pre-disease performance |
1 |
Physically strenuous activity is restricted |
2 |
Ambulatory and capable of all self-care, but no work activities |
3 |
Capable of only limited self-care. Confined to bed more than 50% of time. |
4 |
Cannot carry on any self-care. Bed ridden all the time |
5 |
Dead |
Table 1 Assessment of patient performance status (PST) by scale of the Eastern Cooperative Oncology Group (ECOG)27
Laboratory testing of the patients included serum albumin (gm/dl), bilirubin (mg/dl) prothrombin time in seconds (PT), International normalized ratio (INR), aspartate amino transferase (AST) (u/l), alanine amino transferase (ALT) (u/l), 4th generation ELISA for HCV antibody, quantitative PCR for HCV RNA , serum alpha fetoprotein level (ng/ml), serum creatinine (mg/dl). All the blood samples of patients were received in EDTA anti-coagulated tubes and processed within one hour using the automated hematology analyzer (Mindray BC-2800). From the analyzer generated reports, PLC, MPV& PDW, were recorded. The normal ranges were (150-450×109/L, 7.4 - 10.4 fl and 9.5-14.5 %) respectively. Radiological investigations included abdominal ultrasound, multiphasic contrast enhanced CT (MPCT) and or Dynamic contrast enhanced MRI of the abdomen for non- invasive diagnosis of HCC in cirrhotic patients according to EASL criteria.28.Metastatic workup including CT of brain, chest and pelvis & bone scan were done. Barcelona clinic liver cancer (BCLC) staging depending on HCC burden, Child- Turcotte -Pugh (CTP) class and ECOG performance status (PST), was done for all studied HCC cases.29 BCLC stage 0 & A were considered early stages while BCLC stages B, C & D were considered late stages HCC. Liver cirrhosis was diagnosed through liver histology or a combination of radiographic, clinical, and laboratory findings. CTP class & score, MELD score and ALBI score & grade, were calculated for all participating patients using MDCalc application.
Ethical approval
The study protocol was reviewed and approved by the ethical committee at Faculty of Medicine, Mansoura University, Mansoura, Egypt. The approval code was R.22.03.1658.R1-2022/03/24.Written informed consents were obtained from all participating patients after assuring confidentiality.
Sample size: Sample size calculation was based on prognostic value of platelet distribution width for cases with hepatocellular carcinoma retrived from previous research.30 Using G power program version 3.1.9.4 to calculate sample size based on expected difference of 61.8% of disesae free survival perecntage using 2-tailed test, α error =0.05 and power = 90.0%, the total calculated sample size was 24 cases (Figure 2).
Statistical analysis
Data was analyzed using SPSS (statistical package for social sciences) version 22. Qualitative data was presented as number and percent, Quantitative data was tested for normality by Shapiro-Wilk test then described as mean and standard deviation for normally distributed data and median and range for non-normally distributed. The appropriate statistical test was applied according to data type with the following suggested tests: Chi-Square for categorical variable, Student t test, paired t test, Mann Whitney U test and Wilcoxon signed rank test. The Spearman's rank-order correlation was used to assess the strength and direction of the association between two continuous variables or between one continuous variable and one ordinal variable. ROC curve was used for estimation of cut off values of platelet indices which might predict HCV related HCC and late BCLC stage. Results were considered statistically significant if p value was ≤ 0.05.
Table 2 shows the baseline characteristics of the enrolled patients of both groups. There was significantly older age and higher number of males in group 1 than group 2. The platelet count was significantly lower in group 1 than group 2. There was insignificant difference between both groups regarding MPV & PDW. The serum levels of transaminases were significantly higher in group 1 than group 2.Serum albumin was significantly lower in group 1 than group 2, while serum bilirubin was significantly higher in group 1 than group 2. There was insignificant difference between both groups regarding INR & serum creatinine. Median serum level of AFP was significantly higher in group 1 than group 2. CTP class A was significantly lower while CTP class B & C were significantly higher in group 1 than group 2. CTP score, MELD score & ALBI grade were significantly higher in group 1 than group 2. ECOG PS was significantly worse in group 1. There was insignificant difference between both groups regarding the presence of type II DM. PVT was significantly more common in group 1 than group 2. History of direct acting antiviral (DAA) therapy for chronic HCV was significantly lower in group 1 than group 2. There was insignificant difference between both groups regarding the post-DAA therapy duration.
Variable |
Group1 |
Group 2 |
P |
Age (years) (Mean ± SD) |
62.11 ±7.23 |
56.53 ±9.32 |
0 |
Males |
120 |
52 |
0 |
PLC (Mean± SD)109/L |
127.8 ±78.1 |
156.6 ±80.6 |
0.003 |
MPV (Mean ± SD) |
10.34 ±1.74 |
10.21 ±1.19 |
0.5 |
PDW (Mean ± SD) |
15.43 ± 2.22 |
15.14 ± 1.52 |
0.25 |
ALT (Mean ± SD) |
52.48 ± 37.23 |
30.24 ± 20.56 |
0 |
AST( Mean ± SD ) |
76.06 ± 59.86 |
34.99±23.51 |
0 |
Albumin (Mean ± SD) |
3.47 ± 0.55 |
3.84 ± 0.61 |
0 |
INR (Mean ± SD) |
1.21±0.21 |
1.21±0.25 |
0.9 |
Bilirubin (Mean ± SD) |
1.67 ± 1.35 |
1.16 ± 0.96 |
0 |
Creatinine (Mean ± SD) |
1.03 ± 0.37 |
1.07 ± 0.24 |
0.25 |
AFP: Median (range) |
172(1.2-80000) |
4.3(.6-152) |
0 |
CTP score (Mean ± SD) |
6.97± 1.77 |
6.02± 1.7 |
0 |
CTP class A |
77 (53.4%) |
83 (71.6%) |
0.013 |
MELD score (Mean ± SD) |
10.97 ± 3.95 |
10.04 ± 3.68 |
0.048 |
ALBI grade (Mean ± SD) |
2.08 ± 1.79 |
1.55 ± 0.65 |
0.003 |
PS (Mean ± SD) |
1.27±0.79 |
0.39 ± 0.69 |
0 |
Type II DM Yes |
47 |
32 |
0.343 |
PVT Yes |
56 (38.9%) |
4 (3.5%) |
0 |
DAA Yes |
79(55%) |
88(77.2%) |
0 |
Post DAA years (Mean ± SD) |
4.42± 2.12 |
4.52 ±2.01 |
0.774 |
Table 2 Baseline characteristics of the enrolled patients of both groups
Table 3 shows the baseline characteristics of the enrolled patients of group I (HCC). DAA treatment for chronic HCV was received and sustained virological response (SVR) was achieved in 55% of the cases while 45% of them did not receive DAA. The post DAA duration till the diagnosis of HCC was 4.42± 2.12 years. HCC was diagnosed through adherence to the surveillance program in 7% and accidently discovered in 93% of cases. HCC presented as a single nodule in 46.5%, multiple nodules in 45.1 % and diffuse form in 8.4% of cases. At presentation, HCC diameter was 6.42± 3.98 cm, 18.2% had HCC diameter < 3cm & 81.8% had HCC diameter ≥ 3cm, malignant PVT was present in 38.9% & distant metastases were present in 6.25% of patients. Regarding BCLC staging, HCC was diagnosed at a very early stage (stage 0) in 3.5%, early stage (stage A) in 24.3%, intermediate stage (stage B) in 23.6%, advanced stage (stage C) in 38.9% and terminal stage (stage D) in 9.7% of cases. Median serum level of AFP was 172 ng /ml. It was ≥ 200 ng /ml in 48.6% & < 200 ng/ml in 51.4% of HCC cases. Low PLC was present in 71.6% of HCC cases while normal PLC was present in 28.4% of them. PDW was ≥ 14.5% in 79 % of HCC cases while it was < 14.5% in 21% of them. MPV was ≥ 10.4 fl in 52.1% of HCC cases while it was < 10.4 fl in 47.9% of them.
Variable |
value |
DAA Yes |
79(55%) |
Post DAA years (Mean ± SD) |
4.42± 2.12 |
Discovery |
10 /134 (7/93%) |
HCC number |
67/65/12(46.5/45.1/8.4%) |
HCC diameter (Mean± SD) |
6.42± 3.98 cm |
Malignant PVT (Yes/ No) |
56/88 (38.9 /61.1%) |
Distant Mets(Yes/ No) |
9 /135 (6.25/93.75%) |
BCLC |
5 (3.5%) |
AFP (ng/ml) |
172(1.2-80000) |
PLC: ≤150/ >150 109/L |
103/ 41 (71.6/28.4%) |
PDW % : ≥14.5 / <14.5 |
114/30(79/21%) |
MPV (fl): ≥10.4/<10.4 |
75 /69 (52.1/47.9%) |
Table 3 Baseline characteristics of the enrolled patients of group I (HCC)
Table 4 shows a comparison of HCC patients with normal PLC to those with low PLC. HCC cases with normal PLC had larger HCC diameter than those with low PLC. Malignant PVT was present in 45% of HCC cases with normal PLC while it was present in 35.9% of those with low PLC. Extra-hepatic metastases were present in 2.4% of HCC cases with normal PLC while they were present in 7.7% of those with low PLC. Regarding BCLC staging, very early stage HCC (stage 0) was present in 4.9 % of cases with low PLC and no case was diagnosed at this stage in those with normal PLC. Early stage HCC (stage A) was present in 17.5% of cases with normal PLC and 27.5% of those with low PLC. Intermediate stage HCC (stage B) was present in 30% of cases with normal PLC and 21.6 % of those with low PLC. Advanced stage HCC (stage C) was present in 40% of cases with normal PLC and 38.2 % of those with low PLC. Terminal stage HCC (stage D) was present in 12.5 % of cases with normal PLC and 7.8 % of those with low PLC. There were insignificant differences regarding the median serum level of AFP, CTP score, MELD score & ALBI grade in cases with normal PLC and those with low PLC. Figure 3 shows that regarding HCC cases; PLC at cut off value of 134.5×109/L predicted late BCLC stage; with sensitivity of 44.1%, specificity of 82.5%, AUC of 0.65 & P = 0.006.
Variable |
PLC ≥150×109/L n=41(28.4%) |
PLC <150×109/L n=103(71.6%) |
P value |
HCC diameter |
6.65(2-15) cm |
5(1.4-16) cm |
0.025 |
Malignant PVT |
19(45%) |
37(35.9%) |
0.189 |
Extra-hepatic metastasis |
1(2.4%) |
8(7.7%) |
0.305 |
BCLC |
0% |
4.9% |
0.323 |
AFP: Median (range) |
538(1.9-80000) |
103(1.2-74310) |
0.085 |
CTP score (Mean ± SD) |
6.65± 1.96 |
6.82± 1.69 |
0.604 |
MELD score (Mean ±SD) |
10.18 ± 3.51 |
11.26 ± 4.06 |
0.141 |
ALBI grade (Mean ±SD) |
2 ± .55 |
1.89 ± 1.69 |
0.291 |
Table 4 Comparison of HCC patients with normal PLC to those with low PLC
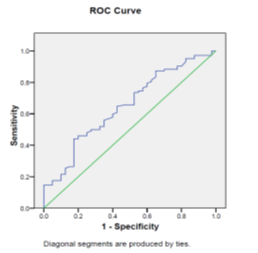
Figure 3 ROC curve shows that regarding HCC cases; PLC at cut off value of 134.5×109/L predicted late BCLC stage; with sensitivity of 44.1%, specificity of 82.5%, AUC of 0.65 & P = 0.006.
Table 5 also shows a comparison of the HCC patients with PDW ≥ 14.5% (79% of the cases) versus patients with PDW < 14.5 % (21% of the cases). There was no significant difference regarding the diameter of the largest HCC nodule in HCC cases with PDW ≥ 14.5% than those with PDW < 14.5 %. Malignant PVT was present in 35.9% of HCC cases with PDW ≥ 14.5% while it was present in 50 % of those with PDW < 14.5 %. Extra-hepatic metastases was present in 7% of HCC cases with PDW ≥ 14.5% while it was present in 3.3% of those with PDW < 14.5 %. Regarding BCLC staging, very early stage (stage 0) was present in 3.2 % of HCC cases with PDW ≥ 14.5% and in 8% those with PDW < 14.5 %. Early stage (stage A) was present in 25.5% of HCC cases with PDW ≥ 14.5% and 8 % of those with PDW < 14.5 %. Intermediate stage (stage B) was present in 26.6 % of HCC cases with PDW ≥ 14.5% and 24 % of those with PDW < 14.5 %. Advanced stage (stage C) was present in 39.4 % of HCC cases with PDW ≥ 14.5% and 44 % of those with PDW < 14.5 %. Terminal stage (stage D) was present in 5.3 % of HCC cases with PDW ≥ 14.5% and 16 % of those with PDW < 14.5 %. There were no significant differences regarding the serum level of AFP, CTP score, MELD score &ALBI grade in HCC cases with PDW ≥ 14.5% and those with PDW < 14.5 %.
Variable |
PDW ≥ 14.5 % n=114(79%) |
PDW < 14.5% n=30 (21%) |
P value |
HCC diameter in cm |
5(1.4-15) |
5.3(1.9-16) |
0.6 |
Malignant PVT |
41(35.9%) |
15(50%) |
0.237 |
Extra-hepatic metastasis |
8(7%) |
1(3.3%) |
0.672 |
BCLC |
3.2% |
8% |
0.1 |
AFP: Median (range) |
78.8(1.2-74310) |
424(3.3-80000) |
0.074 |
CTP score (Mean ± SD) |
6.88±1.87 |
6.59±1.6 |
0.353 |
MELD score (Mean ±SD) |
11.26±3.61 |
10.7±4.17 |
0.424 |
ALBI grade (Mean ±SD) |
1.98±.55 |
1.84±.52 |
0.158 |
Table 5 Comparison of HCC patients with PDW ≥ 14.5 % to those with PDW < 14.5%
Figure 4 shows that PDW at a cut-off 15.05% may predict HCV related HCC; with sensitivity of 62.2%, specificity of 58.1%, AUC of 0.58, P = 0.043 & asymptotic 95% confidence interval (0.503- 0.654).
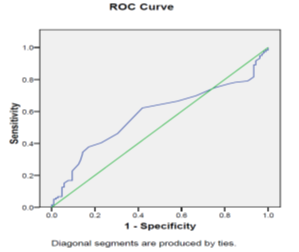
Figure 4 ROC curve shows that PDW at cut off value of more than 15.05% can predict HCC; with sensitivity of 62.2%, specificity of 58.1%, AUC of 0.58, p = 0.043 & asymptotic 95% confidence interval (0.503- 0.654).
Table 6 also shows a comparison of the HCC burden in patients with MPV ≥ 10.4 fl (52.3 % of the cases) versus patients with MPV< 10.4 fl (47.7% of the cases). There was no significant difference regarding the diameter of the largest HCC nodule in HCC cases with MPV ≥ 10.4 fl and those with MPV < 10.4 fl. Malignant PVT was present in 42.6% of HCC cases with MPV ≥ 10.4 fl while it was present in 34.7% of those with MPV< 10.4 fl. Extra-hepatic metastases was present in 6.6% of HCC cases with MPV ≥ 10.4 fl while it was present in 5.7 % of those with MPV< 10.4 fl. Regarding BCLC staging, very early stage HCC (stage 0) was present in 1.5 % of HCC cases with MPV ≥ 10.4 fl and in 6.6% those with MPV< 10.4 fl. Early stage HCC (stage A) was present in 29.9% of HCC cases with MPV ≥ 10.4 fl and 18 % of those MPV < 10.4 fl. Intermediate stage HCC (stage B) was present in 17.9 % of HCC cases with MPV ≥ 10.4 fl and 32.8 % of those with MPV < 10.4 fl. Advanced stage HCC (stage C) was present in 41.8 % of HCC cases with MPV ≥ 10.4 fl and 36.1 % of those with MPV < 10.4 fl. Terminal stage HCC (stage D) was present in 9 % of HCC cases with MPV ≥ 10.4 fl and 6.6 % of those with MPV < 10.4 fl. The serum level of AFP was significantly lower in HCC cases with MPV ≥10.4 fl than those with MPV <10.4fl. There were insignificant differences regarding CTP score, MELD score &ALBI grade in HCC cases with MPV ≥10.4 fl and those with MPV <10.4fl.
Variable |
MPV ≥10.4fl n=75(52.1 %) |
MPV <10.4fl n=69(47.9%) |
P value |
HCC diameter: |
5 (1.6-15) |
5.1(1.4-16) |
0.641 |
Malignant PVT |
32(42.6%) |
24(34.7%) |
0.148 |
Extra-hepatic metastasis |
5(6.6%) |
4(5.7%) |
0.865 |
BCLC |
1.5% |
6.6% |
0.1 |
AFP: Median (range) |
39.5(1.8-80000) |
406(2.3-74310) |
0.013 |
CTP score (Mean ± SD) |
6.88±1.87 |
6.59±1.6 |
0.353 |
MELD score (Mean ±SD) |
11.26±3.61 |
10.7±4.17 |
0.424 |
ALBI grade (Mean ±SD) |
1.98±.55 |
1.84±.52 |
0.158 |
Table 6 Comparison of HCC patients with MPV ≥ 10.4 % to those with MPV < 10.4%
Table 7 shows the correlations between the PIs and BCLC, serum AFP level, CTP score, MELD score &ALBI grade. There was significant positive correlation of PLC with BCLC stage (Figure 5). There was significant negative correlation of PLC with MELD score. There were insignificant correlations of PLC with serum AFP level, CTP score & ALBI grade. There were insignificant correlations of PDW with BCLC stage, serum AFP level, CTP score, MELD score & ALBI grade. There was significant negative correlation of MPV with serum AFP level (Figure 6). There were insignificant correlations of MPV with BCLC stage, CTP score, MELD score & ALBI grade.
Variable |
PLC (109/L) |
PDW% |
MPV(fl) |
|||
r |
p |
r |
p |
r |
p |
|
BCLC stage |
0.18 |
0.027 |
-0.13 |
0.15 |
-0.05 |
0.9 |
AFP (ng/ml) |
0.18 |
0.18 |
-0.1 |
0.25 |
-0.19 |
0.03 |
CTP score |
-0.118 |
0.16 |
-0.12 |
0.18 |
0 |
0.9 |
MELD score |
-0.18 |
0.027 |
-0.13 |
0.15 |
0.07 |
0.4 |
ALBI grade |
0.016 |
0.85 |
-0.06 |
0.52 |
0.09 |
0.31 |
Table 7 Correlations between PIs and BCLC, AFP level, CTP score, MELD score &ALBI grade
Table 8 shows a comparison between HCC cases characterized by PLC < 150×109/L with MPV ≥ 10.4 fl and HCC patients with other PIs. Serum AFP level was significantly lower in HCC cases characterized by PLC < 150×109/L with MPV ≥ 10.4 fl than HCC patients with other PIs. However, there was no significant difference between them regarding HCC diameter, malignant PVT, extra-hepatic metastasis, BCLC stage, CTP score, MELD score & ALBI grade.
Variable |
PLC < 150 109/L + MPV ≥ 10.4 fl |
P value |
|
Yes (n=44) |
No (n=75) |
||
HCC diameter (cm) |
4.7(1.6-15) |
5.5(1.4-16) |
0.088 |
Malignant PVT |
15(34.1%) |
30(40%) |
0.523 |
Extra-hepatic mets |
3(7%) |
4(5.8%) |
0.838 |
BCLC |
2.3% |
5.3% |
0.573 |
AFP: Median |
30ng/ml |
311ng/ml |
0.007 |
CTP score: mean ± SD |
6.86±1.74 |
6.77±1.75 |
0.795 |
MELD score: mean ± SD |
11.17±3.67 |
11.01±4.13 |
0.842 |
ALBI grade: mean ± SD |
1.93±.53 |
1.9±.56 |
0.846 |
Table 8 Comparison between HCC cases characterized by PLC < 150×109/L associated with MPV ≥ 10.4 fl and HCC patients with other PIs
Table 9 shows a comparison between early and late BCLC stages. HCC diameter was significantly smaller in early than late BCLC stage. PLC was significantly lower in early than late BCLC stage. MPV was significantly larger in early than late BCLC stage. There was insignificant difference between early & late BCLC stages regarding PDW. Median serum AFP level was significantly lower in early than late BCLC stage. CTP score was significantly lower in early than late BCLC stage. CTP class A was significantly higher in early than late BCLC stage while CTP classes B & C were significantly lower in early than late BCLC stage. MELD score was significantly lower in early than late BCLC stage. There was insignificant difference between early & late BCLC stages regarding ALBI grade.31
Variable |
Early BCLC stages |
Late BCLC stages |
P value |
HCC diameter (Mean ± SD) cm |
2.88 ±0.82 |
7.8 ±3.87 |
0 |
PLC (Mean ± SD) 109 /L |
98435 ±48101 |
138313 ±84674 |
0.001 |
MPV (Mean ± SD) |
10.89 ±1.97 |
10.13 ±1.61 |
0.025 |
PDW (Mean ± SD) |
15.69 ±2.24 |
15.34 ±2.22 |
0.456 |
AFP (ng/ml): median |
30(2.3-9487) |
412(1.2-80000) |
0.016 |
CTP score |
6.13 ±1.22 |
7.04 ±1.88 |
0.001 |
CTP class: A/B/C |
30/10/0 (75/25/0%) |
48/44/12 (46.2/42.3/11.5%) |
0.05,0.01,0.01 |
MELD score (Mean ± SD) |
9.63 ±2.59 |
11.46 ±4.24 |
0.003 |
ALBI grade: median (range) |
2(1-3) |
2(1-3) |
0.358 |
Table 9 Comparison of PIs, serum AFP level, MELD score CTP score, CTP class &ALBI grade in early versus late BCLC stage
Table 10 shows that gender, PS, AFP, PLC, ALBI grade and MELD score were significant predictors of HCV related HCC. It also shows that age, PDW, MPV and CTP score were not significant predictors of HCV related HCC.
Variable |
B |
Odds Ratio |
95% C.I. |
P value |
|
Lower |
Upper |
||||
Age |
- |
0.96 |
0.905 |
1.024 |
0.22 |
Gender |
- |
0.1 |
0.029 |
0.324 |
0.001 |
PLC |
0 |
1 |
1 |
1 |
0.02 |
PDW |
0.119 |
1.12 |
0.88 |
1.43 |
0.334 |
MPV |
- |
0.98 |
0.7 |
1.35 |
0.909 |
CTP score |
0.373 |
1.45 |
0.77 |
2.7 |
0.25 |
MELD score |
0.267 |
1.3 |
1 |
1.7 |
0.05 |
ALBI grade |
-1.33 |
0.26 |
0.079 |
0.87 |
0.029 |
PS |
-1.6 |
0.2 |
0.074 |
0.549 |
0.002 |
AFP |
- |
0.98 |
0.978 |
0.997 |
0.013 |
Table 10 Multivariate logistic regression analysis of HCV related HCC predictors
The present study showed that PLC was significantly lower in HCV related HCC than HCV related cirrhosis. Low PLC was present in 71.6 % of HCV related HCC cases, while 28.4 % of them had normal PLC. On multivariate analysis, PLC was significant predictors of HCV related HCC. HCC is diagnosed at early BCLC stages in patients with low PLC (32.4% of the cases were BCLC 0 or A) more commonly than patients with normal PLC (17.5% of the cases were BCLC 0 or A) and diagnosed at late BCLC stages in patients with normal PLC (82.5% of the cases were BCLC B, C or D) more commonly than patients with low PLC (67.6% of the cases were BCLC B, C or D). Also, at diagnosis; the diameter of the HCC was significantly larger in patients with normal PLC than those with low PLC. There was significant positive correlation of PLC with BCLC stage and PLC was significantly higher in late than early BCLC stage. A recent study also reported that the frequency of vascular invasion & extra-hepatic spread as well as BCLC stage were significantly lower in patients with low PLC. They also reported that patients with low PLC had a significantly smaller diameter of the largest tumor at diagnosis when compared to patients with a normal PLC.32
The present study showed that, regarding HCV related HCC cases, PLC at cut off value of 134.5×109/L predicted late BCLC stages. These findings may be explained by the deceiving effect of normal PLC, which is usually associated with preserved liver function as evidenced by significant negative correlation of PLC with MELD score in the present study. The presence of normal PLC, low MELD score besides the successful clearance of HCV by the highly effective DAAs (55% of HCC cases in this study have received DAAs & achieved SVR) may deceive the patient as well as the health care provider regarding the adherence to surveillance program aiming at early detection of HCC in HCV patients with cirrhosis. This resulted in underuse of the surveillance tools. On the other hand, patients with Low PLC may be more advised to repeat abdominal US &AFP semi-annually by their health care provider. The present study showed that only 7% of the cases have been diagnosed through the surveillance program. The accidental discovery of HCC in the present study occurred in 93% of HCC cases which resulted in diagnosis of HCC at early BCLC stages in only (27.8%) of cases, which is much lower than reported (40%) (18), while the remaining cases (72.2%) were diagnosed at late BCLC stages .This mode of HCC discovery in the present study, resulted in diagnosis of HCC while 81.2% of the patient had a large tumor diameter (≥ 3cm) and 38.9% of them had malignant PVT.
The significantly higher PLC in late BCLC stage may be explained by the increase of hepatic thrombopoietin (THPO) production during the transition from cirrhosis to HCC. It was reported that THPO was produced by malignant hepatocytes and it controlled the number and activity of PLTs.33 Also, it is known that activated PLTs secret several growth factors that lead tumor progression, like the platelet-derived growth factor, the vascular endothelial growth factor and serotonin which has direct tumor-promoting effects on HCC cells by activating downstream targets of the mTOR.34 PLTs are also involved in tumor spread through increasing the endothelial permeability and allowing the extravasation of malignant cells. These findings denote the presence of tumor–platelet axis in HCC; characterized by a mechanism in which the tumor activates platelets and platelets enhance tumor progression.14
In the present study; there was insignificant difference between both groups regarding MPV & PDW. This may be explained by the finding that the MPV and PDW positively correlated with liver fibrosis in HCV-infected patients.35 The present study showed that platelet activation was present in 79% of HCV related HCC cases as evidenced by increased PDW. PDW is a known specific marker for platelet activation because it does not increase during simple platelet swelling.36 It also showed that the cut off value of PDW which may predict HCV related HCC was 15.05%. In the present study; there was no significant difference between HCV related HCC cases with high PDW & those with normal PDW regarding BCLC stage, AFP, CTP score, MELD score and ALBI grade. Also, there was no significant difference between early and late BCLC stages regarding PDW. Recent study which involved Chinese patients with different risk factors for HCC reported a significant increase in the PDW value as BCLC stage progressed from A to B to C (38). In the present study, MPV was increased (≥ 10.4 fl) in 52.1% of HCV related HCC cases while it was <10.4fl in 47.9% of them. MPV was significantly larger in early than late BCLC stage.
There was significant negative correlation of MPV with serum AFP level .The serum level of AFP was significantly lower in HCC cases with increased MPV than those with normal MPV <10.4fl. Also, in the present study, HCV related HCC patients who had both low PLC and increased MPV had a significantly lower serum AFP level. MPV is a measure of platelet size and its increase is considered a surrogate marker of platelet activation.37 The findings in the present study regarding the changes in MPV can be explained by the fact that cancer-associated increase in PLTs activation and subsequent PLTs exhaustion is the cause of the decrease in MPV in late BCLC stages.38,39 There were insignificant differences regarding BCLC stage, CTP score, MELD score &ALBI grade in HCC cases with MPV ≥10.4 fl and those with MPV <10.4fl. Recent research reported that HCC patients with an MPV ≥11 fl at diagnosis had lower CTP stage and MELD scores.32 Their study involved patients with different risk factors for HCC & patients who have already received different treatment modalities for HCC. In the present study, multivariate logistic regression analysis showed that that male gender, PS, AFP, PLC, ALBI grade and MELD score were significant predictors of HCV related HCC. It also shows that age, PDW, MPV and CTP score were not significant predictors of HCV related HCC. This finding is in agreement with the recently developed aMAP score, which involved age, male gender, albumin-bilirubin and platelets (31) but the present study has added PS, AFP, ALBI grade and MELD score as significant predictors of HCV related HCC and denied age as a significant predictor. aMAP score study included Asian chronic hepatitis B , Caucasian chronic hepatitis B, chronic hepatitis C infected and non-viral hepatitis patients. The present study included only Egyptian patients with HCV cirrhosis, which may explain the differences in the predictors of HCC. The findings of the present study may attract the attention towards the possible benefits of anti-PLTs drugs in management of HCC especially in patients with normal PLC & increased PDW. Recent study reported that anti-PLTs therapy had preserved liver function and prevented HCC progression.40
The strengths of the present study were the presence of cirrhotic patients as a control group & the inclusion of only naïve HCV related HCC patients. The limitations of the present study were the small number of patients and lack of control group of healthy individuals. More research is required to confirm these findings and to demonstrate the possible beneficial effects of anti-PLTs regarding the prevention of HCV related HCC and treatment of certain subgroups of this tumor.
PLC was significant predictors of HCV related HCC. HCV related HCC patients with normal PLC had larger tumor diameter. PLC positively correlated with BCLC stage. In HCV related HCC cases, PLC at cut off value of 134.5×109/L may predict late BCLC stages. PDW at cut off 15.05% may predict HCV related HCC. MPV was significantly smaller in late than early BCLC stages & it negatively correlated with serum AFP level. MPV was not significant predictors of HCV related HCC.
None.
The authors declare no conflicts of interest.

©2023 Shabana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.