eISSN: 2373-6372


Review Article Volume 15 Issue 4
Department of Gastroenterology & Hepatobiliary Sciences, Fortis Memorial Research Institute, India
Correspondence: Gourdas Choudhuri, Department of Gastroenterology & Hepatobiliary Sciences, Fortis Memorial Research Institute, India, Tel +919650643222
Received: June 11, 2024 | Published: July 25, 2024
Citation: Choudhuri G, Kalel SM, Sharma ZD, et al. MASLD- Global prevalence, pathophysiological processes and management pathways- tackling a complex problem. Gastroenterol Hepatol Open Access. 2024;15(4):74‒88. DOI: DOI: 10.15406/ghoa.2024.15.00585
Metabolic dysfunction associated steatotic liver disease (MASLD), is characterised by deposition of fat in liver which can be associated with necroinflammation and fibrogenesis, which may progress to liver cirrhosis or hepatocellular carcinoma (HCC). This review intends to highlight the increasing prevalence, increasing data on genetic predisposition, gut microbiome and pathophysiological processes involved in the complex interplay for development of MASLD. The complex pathways also highlight the association of MASLD with cardiometabolic disorders like diabetes, atherosclerotic heart disease and dyslipidaemia particularly for hypertriglyceridemia. It also reviews briefly the diagnostic tools available in assessing the disease as well as lays outlay for the management of MASLD by various means including lifestyle interventions, pharmacotherapy and surgical options. Endoscopic and surgical weight management therapies have also been shown to be effective in MASLD. However, access and acceptability remain poor for these weight reduction methods. The developments in the integrated management of MASLD have been fairly encouraging with many programs encompassing lifestyle modifications and pharmacological interventions together. Further well-designed long-term prospective studies should be undertaken to generate evidence with definitive results.
Keywords: MASLD, fibrosis, lifestyle modifications, weight loss, endobariatrics, pharmacotherapy, GLP-1-GIP co-agonist, FGF analogs, THR-ß agonist, few PPAR-α/δ agonists, FXR agonist, and DGAT2i/ACCi
Metabolic dysfunction associated steatotic liver disease (MASLD) or more appropriately termed Metabolic (dysfunction) associated fatty liver disease or ‘MAFLD’ is a disease which encompasses multiple pathophysiological processes in a genetically susceptible individual leading the changes that trigger significant inflammation and fibrosis in the liver. It was previously named as Non-alcoholic fatty liver disease or NAFLD and is still popular with that name. It is now the most common chronic liver disease (CLD) with an estimated worldwide prevalence of approximately 30%.1-5 MASLD usually leads to or can be associated with necroinflammation in the liver which is called Metabolic dysfunction associated steatohepatitis (MASH) which may lead to cirrhosis and hepatocellular carcinoma (HCC).6,7 Hepatic steatosis >5% of hepatocytes in the absence of alcohol abuse or any other hepatic disease associated with ballooning and inflammation in the liver biopsy confirms MASH.4,5 It may lead to advanced hepatic fibrosis. MASH associated cirrhosis is currently the second indication for liver transplantation and is likely to become the leading one by the next decade, both globally and in India.8,9 MASLD and MASH is also a marker of metabolic syndrome, and is associated with higher overall mortality with chronic kidney disease and poorer cardiovascular outcomes.10,11 MASLD has a high all-cause-mortality (25.56 per 1000 person-years).12 However, though MASH is now considered to be a serious condition associated with severe morbidity and mortality, robust and viable diagnostic tools and treatment for cure are still evolving. The role of the current review is to elaborate rising prevalence, touching upon genetic predisposition, and elaborating on lifestyle interventions in management of MASLD. It also delves into emerging therapy targets and drug classes, investigating their efficacy in the treatment of patients with MASH.
MASLD and MASH Burden: Global and India
Getting a true prevalence of MASLD and MASH is difficult because of lack of a single screening test. Liver biopsies being the gold standard are difficult to acquire and there may be limitations to differentiate MASLD and MASH based on non-invasive parameters alone.
The estimated overall global prevalence of MASLD in adults is ~32%. Various studies from the USA, Europe, and Asia have reported a prevalence of approximately 22%, 37%, and 31%, respectively in the general population.3 The prevalence of MASLD shows wide variation across India ranging from 9% to 53%.13-15 This wide variation is possibly due to urban-rural divide but this may also be challenged in near future.13,16 40-68% of MASLD patients will have progressed to definite biopsy-confirmed MASH. In Indian patients, biopsy-proven MASH has been found in >60% of patients and advanced fibrosis (≥F3) in 29-35% of patients usually at the time of diagnosis as most of the patients are picked up when liver function tests are deranged.17-19 This may also reflect a referral bias as biopsy is usually done in patients with more advanced stage of disease.
There are age and gender differences as far as prevalence is concerned. It is higher in men as compared to women (~39% vs ~25%). Post Menopause, this difference tends to get abolished possibly due to hormonal protective effects prior to that.20 The progression of MASH is also considered to be faster in elderly women.21 Diabetics have one of the highest prevalence of MASLD.22 People with obesity also have a higher prevalence of MASLD (90%) and MASH (30%) compared to non-obese (25-30%%).23-25 Indian obese patients also have a high prevalence of MASLD, almost more than 80%.13 MASH was diagnosed in ~60% of those with both diabetes and obesity. MASH (non-viral factor) contributes almost equally as viral etiological factor for HCC [MASH >17% of patients, Hepatitis B (HBV) >20% and Hepatitis C (HCV ~17%)].26 A systematic analysis of the Global Burden of Disease Study has shown that the age-standardized prevalence of compensated cirrhosis has doubled (33%) and decompensated cirrhosis tripled (55%) due to MASH compared to all other causes.27 MASH-associated HCC and decompensated cirrhosis are now one of the leading indications for liver transplantation in India.13
Different phases of MASLD: Progress from healthy to cirrhosis MASLD
As explained above, MASLD is a complex disease which involves various pathophysiological mechanisms occurring leading to development of fibrosis and subsequent HCC.28 It usually begins with simple steatosis leading to development of inflammation and fibrosis (Figure 1).28
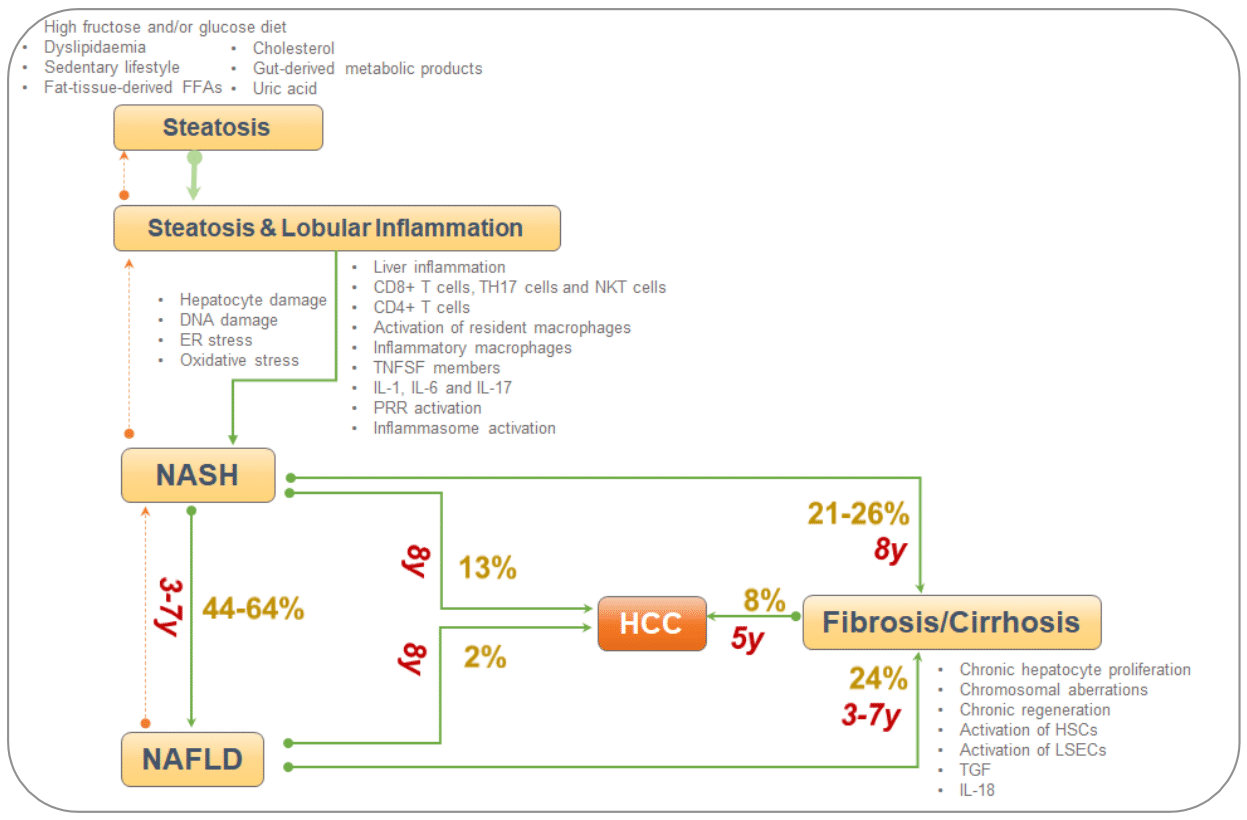
Figure 1Phases of MASLD (FFA-free fatty acids, IL-interleukin, HSC-hematopoietic stem cell, NKT-, ER-Endoplasmic reticulum, DNA-deoxyribonucleic acid, HCC-hepatocellular carcinoma, liver sinusoidal endothelial cells, NKT, natural killer; T, cells; y, years).
Role of Microbiome in MASH
Humans have a wide spectrum of commensal microbiota.29-31 It has been well established that human microbiome plays an important role in various metabolic pathways in diseased states. Though inconsistently reported dysbiosis has also been reported in pathways of MASLD and MASH, both in its inception as well as progression (Figure 2).33
Studies done in patients with diabetics show a various interplay of dysbiosis and alterations in metabolic pathways leading to hepatic inflammation and fibrosis.31 There is marked prominence of bacteroids and elevated fecal short chain fatty acids in obese patients.
Mechanisms by which the gut microbiota impact the progression to MASH are being explored and a few have been described using experimental data (Table 1).35 The compositional changes and mechanisms currently in pathogenesis of MASH and MASLD are inconsistent and not validated outside of research. Priobiotics, prebiotics and synbiotics all have been investigated in MASLD as well as Fecal microbiota transplantation.36-47 These methods have shown to alter endotoxemia as well as intestinal permeability which leads to improvement in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels, hepatic inflammation, increase the copiousness of Faecal bacterium prausnitzii and Bifidobacterium all of which were beneficial in MASH therapy. Various studies have also looked into role of FMT in modulation of inflammation in MASLD and MASH with limited success, currently at best as an experimental data.
|
Effect of Dysbiosis on gut and Liver |
Effect on pathogenesis of MASH |
|
SCFA ¯ SCFA production Disturb the integrity of the intestinal barrier |
#Loss of intestinal wall integrity and Dysregulated hepatic metabolism # Increased hepatic steatosis, inflammation |
|
FXR Insufficient activation of FXR thus reduced activity |
Encourages hepatic steatosis and insulin resistance, as well as negative feedback, inhibits bile acid synthesis |
|
TGR5 Insufficient activation of TGR5 thus reduced activity |
#Increases inflammation and IR |
|
Trimethylamine #¯ choline levels and increasing toxic choline metabolites #Suppresses the activation of liver FXR signaling #Upregulates glucose metabolism and increases IR #Induce the activation of the NF-kB pathway, promote oxidative stress and activate the NLRP3 inflammasome, # the release of inflammatory cytokines (IL-18 and IL-1b) |
Effect of TMAO on MASH is controversial. |
|
LPS LPS deteriorates MASH progression: #Induces hepatic inflammatory response and fibrosis via LPS/TLR4 and NFkB signaling pathways in hepatocytes, HSCs, and Kupfer cells #Activates CD14-TLR4 promoting the release of inflammatory cytokines (NF-kB) #Induces the activation of macrophages and platelets through the TLR4 pathway, thereby eliciting liver damage #Promotes the expression of TGF-β, which induces the transcription of certain pathways promoting hepatic fibrosis #Induces oxidative stress |
Hepatic inflammation, fibrosis, and liver injury |
|
Activation of Inflammasomes NLRP3 #Activates NLRP3 by promoting the entry of PAMPs, DAMPs, and LPS into the portal circulation through the impaired intestinal barrier, resulting in liver inflammation. Instigates IR |
Participates in the transition from MASLD to MASH to hepatic fibrosis through the TLR4-NF-kB signalling pathway |
|
NLRP6 #Deletion of NLRP6 alters the configuration of the intestinal microbiota, resulting in hepatic steatosis and inflammation via TLR4 signaling |
Increased hepatic steatosis |
|
Intestinal dysbiosis # abundance of Escherichia # the production of endogenous alcohol- #the expression of intestinal inflammatory factors and destroys the intestinal barrier, associated with small intestinal bacterial overgrowth, and aggravates intestinal dysbiosis Endogenous alcohol inhibits the TCA cycle and aggravates hepatic TG accumulation and deposition Toxic intermediates of alcohol metabolism (acetaldehyde) impair the function of intestinal tight junction proteins |
Aggravation of hepatic steatosis, inflammation, mitochondrial dysfunction, and liver injury promotes the progress of MASH. |
Table 1 Role of the microbiota in MASH
AMP, antimicrobial peptide; damage-associated molecular patterns, FXR, Farnesoid-X Receptor, GLP,1 glucagon-like peptide 1; LPS, lipopolysaccharide; NF-KB, nuclear factor kappa-B; NLR, Nod-like receptors; PAMPS, pathogen‐associated molecular patterns; SCFA, short chain fatty acids; TG, triglyceride; TLR-toll-like receptors, Treg, Regulatory T cells; TMAO, Trimethylamine N-oxide.
Role of genetics
There is genetic heterogeneity in predisposition as well as progression of MASLD in various individuals. Various studies have shown that steatosis and fibrosis can occur in families occur with a heritability value of ~ 0.5 in age-, gender- and ethnicity-adjusted analysis.48 The risk of accelerated progression in fibrosis scale and cirrhosis is much faster in relatives of patients with MASLD or MASH.49 Genome-wide association have identified various candidate genes which may increase predisposition and progression in MASH namely patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), membrane-bound O-acyltransferase domain-containing 7 gene (MBOAT7), glucokinase regulator (GCKR), hydroxysteroid 17-beta dehydrogenase-13 (HSD17B13).50,51 PNPLA3 gene has been the commonest in this group, found in 15% of the general population, in which a mutation at position 148 is linked to 2.5-fold greater odds of the development of severe steatohepatitis, excessive, inflammation, injury, and scarring; consequently, bearing higher risks of MASH, progression to cirrhosis and HCC.52 Other genes like TM6SF2, GCKR, and MBOAT7 also increase the odds of promoting MASH progression by 1.18- to 1.55-fold but are rarer compared to PNPLA3.53-55 Another variant is HSD17B13 although rare is a protective variant neutralizing some of the deleterious effects of the PNPLA3 gene in people with both gene variants. The risk of MASH was reduced by 14% in presence of the HSD17B13 variant.56 It must be highlighted that MASLD and MASH are essentially a polygenic disease with multiple mutations may happen and lead to a fibrotic progression. It is possible that a polygenic score helps to quantitatively determine the composite risk of disease progression based on the proportion of deleterious and protective genes. Though the evidence is currently limited, it has been theorized that genetic factors also determine the response to MASH therapy e.g. decreased effect of PUFA or fish oil in patients with fatty liver with PNPLA3 variant.57
The practical implications of genetics of MASLD are to be considered keeping in mind the environmental and lifestyle factors. The genetic propensity has been the same in a community pool but it is the environmental factors added to lifestyle features that change within communities leading to development of advanced stages of fibrosis and subsequently cirrhosis.
Lean MASH
First reported in Asia, lean MASH is now an accepted diagnosis worldwide. Individuals with normal BMI (25 kg/m2 in Caucasians and 23 kg/m2 in Asian subjects) manifesting inflammation and fibrosis are considered as Lean MASLD or MASH. Insulin resistance is one of the important pathophysiological processes that leads to development of Lean MASLD. Though Lean MASLD is similar, but not identical to MASLD, current evidence suggests that MASH develops at a faster rate in these patients as compared to obese MASH.58
Incidence
Asian populations tends to develop MASLD and MASH at much lower BMIs as compared to western population (Table 2).59-66 These patients too exhibit the whole spectrum of the histopathological characteristics of MASH.
|
Country |
BMI kg m2 |
N |
Method |
MASLD/MASH(%) |
Risk factor |
|
China (Zhejiang Zhenhai Study) |
<25 |
6905 |
Ultrasound (US) |
7.27 |
|
|
China |
<24 |
2000 |
Physical examination |
18% |
Metabolic syndrome |
|
Korea |
<25 |
3014/29,994 |
Routine health evaluation (incidental) |
12.6% |
|
|
Korea |
<25 |
1487 |
Routine health evaluation (incidental) |
22.4% |
Male gender, WC, TG, IR |
|
Japan |
<25 |
3271 |
Routine health evaluation (incidental) |
15.2% vs 68.5% in obese |
WC, body fat percent |
|
Hongkong |
<23 |
911 |
proton-magnetic resonance spectroscopy |
19.3% vs 61 % in obese |
|
|
India |
<25 |
1911 |
Biopsy |
8.6% |
Higher bicep skin fold thickness |
|
India |
<23 |
150 |
- |
15.3% |
|
Table 2 Prevalence of Lean MASLD/MASH in the Asian Population
BMI, body mass index; CT, computed tomography; HOMA-IR, Homeostatic model assessment – insulin resistance;
Met-S, Metabolic Syndrome; MASLD, nonalcoholic fatty liver disease; TG, triglycerides; US, ultrasound;
WC, waist circumference.
The lean individuals with MASLD were phenotypically distinct: excess subcutaneous fat elevated fasting plasma glucose (FPG) and triglycerides (TGs).65 Metabolic factors such as WC and TGs are predictors of lean MASLD. Apart from these, weight gain in early adulthood is significantly associated with MASLD in lean subjects. There seem to be a different genetic risk factors associated with lean MASLD and MASH.
Outcomes in LEAN MASLD/MASH
Given the different phenotype in Lean MASH, a study in Indian subjects (BMI <23 kg/m2) found the prevalence of biopsy-proven MASH to be ~8%.65 Amongst these subgroups of patients, cirrhosis was found in approximately 2% of patients. The largest and longest series of patients with biopsy-proven MASLD with a mean follow-up time of >19 years found a high rate (19%) of MASLD in lean subjects.59 Though there was no increased mortality in lean compared to overweight subjects, they were at higher risk, for the development of severe liver disease (decompensated liver disease, liver failure, HCC, or cirrhosis).68 Patients who develop more severe disease in lean MASLD group were usually older, had more severe portal inflammation and higher fibrosis staging.60
A study from US made a similar comparison found that lean patients developed significantly more severe lobular inflammation than the non-lean MASLD group.61 The age-adjusted cumulative survival is significantly shorter in patients with lean MASLD as compared to those with non-lean MASLD.71 A European population study showed that lean patients had significantly less severe histological disease; and less advanced fibrosis. There were no significant differences in the prevalence of the PNPLA3 variant. Further follow-up for ~8 years, found no significant difference in hepatic events or survival.62 From UNOS database, A comparison of survival after liver transplant in lean and obese individuals after adjusting for confounders found that all obesity cohorts with MASH had significantly reduced risk of graft and patient loss at 10 years of follow-up compared with the lean BMI cohort.63
Hence, the clinical evidence regarding survival and histological disease severity is not clear in lean MASH vs non lean MASH. We certainly need more data to. Nevertheless, these data do highlight the implication of a genetic link in absence of an excess adiposity in MASLD/MASH pathogenesis and serious negative clinical outcomes.
Risk factors for MASH
The susceptibility and progression of MASH are linked to the interaction between environmental and genetic risk factors. Obesity, older age, female sex, non-African American race/ethnicity, diabetes mellitus, and hypertension are the risk factors that increase the probability of MASH (Table 3).
|
Risk factor |
Mecjanism of interactions |
Illustartive evidence |
|
Diet and extrahepatic milieu |
High‐calorie diets, excessive consumption of sugar |
Subjects with MASLD had a 2 to a 3-fold higher intake of fructose from sugary sweetened beverages than healthy controls |
|
Promotes obesity and excess adipose tissue leading to excess inflammatory cytokine formation. |
||
|
Affects the intestinal microbiome and alters intestinal permeability, exposure to endotoxins sets an inflammatory cascade. |
Fructose consumption increases in liver fat confirmed by MRS.[iii]Its restriction reduces liver fat and de novo lipogenesis in subjects with high baseline fructose consumers compared to an isocaloric diet. |
|
|
Provides excessive FFA to the liver both directly and by promoting IR |
||
|
Metabolic inflexibility, energy imbalance scale causing lipotoxic cell stress |
||
|
Central Adiposity |
Visceral fat is a predictor of hepatic steatosis, hyperinsulinemia, decreased hepatic insulin extraction, and peripheral IR. |
Decreasing visceral fat has also been shown to decrease hepatic IR. |
|
Lipolysis in VAT is more resistant to insulin providing hepatoxic FAs in hyperinsulinaemic states. |
Lean MASH patients may have central adiposity. |
|
|
Metabolic syndrome |
Fatty liver is the hepatic component of the IR syndrome (IRS) |
The risk of hepatic steatosis increased exponentially with each addition of the components of the IRS |
|
The presence of the metabolic syndrome makes it more likely that a patient will have MASH rather than steatosis |
||
|
Obesity |
Lipid-laden hepatocytes act as a reservoir for hepatotoxic agents that are more vulnerable to a successive injury by endotoxins and cytokines in the production of ROS which causes lipid peroxidation and activation of cytokines, stimulating fibrogenesis and causing cell death |
Though MASH can occur in lean subjects, 57-93% of patients are overweight or obese |
|
Adipocytes are endocrine tissue secreting TNF, resistin, leptin, and free fatty acids that may induce/enhance IR |
||
|
A decrease in adiponectin decreased insulin sensitivity |
||
|
Diabetes (Bidirectional) |
IR, oxidative stress, metabolic inflexibility |
Prevalence of MASH among the diabetic population was 37.3% compared to 3-5% in the general population and a significantly high proportion of developing advanced MASH fibrosis (17%) |
|
Hypertension (Bidirectional) |
Inflammation, renin-angiotensin system-sympathetic nervous system activation and insulin resistance, endothelial dysfunction |
Epidemiological evidence shows 49.5% MASLD prevalence in hypertension patients, which is higher than in the general population[vii1.63 times greater risk of MASLD |
|
Shared genes among hypertension, MASLD, fibrosis, and inflammation include LEP, ADIPOQ, AHR and TGFB181 |
Prevalence is 16.5%, 37.5%, and 59.3% in normotensive, pre-hypertensive and hypertensive population |
|
|
Polycystic ovarian syndrome (PCOS) |
The aberrant metabolic and hormonal milieu |
Women with vs without PCOS |
|
Higher proportion had severe ballooning (32 vs 13%, p=0.02), |
||
|
Presence of any fibrosis (84 vs 66%, p=0.06), |
||
|
Presence of advanced fibrosis (16 vs 6%, p=0.10). |
||
|
Age- and BMI-adjusted analysis found >3-fold greater risk of severe hepatocyte ballooning (p=0.03) and 7-fold greater risk of advanced fibrosis (p=0.02). The median age was 5 years younger in advanced fibrosis (40 vs 45 years, p=0.02) |
||
|
Obstructive sleep apnea (OSA) |
May facilitate the progression of hepatic steatosis to MASH. Induced CIH resembling OSA causes hepatocyte injury, increases lipolysis, and oxidative stress, and up-regulates hypoxia-inducible factor 1, which may increase hepatic steatosis, induce necro-inflammation and fibrogenesis |
Three-fold greater risk of MASH in patients with OSA than without OSA after adjusting for confounders (2% vs 0.65%; p<0.0001) |
|
Sarcopenia Muscle and adipose tissue |
Muscle- key metabolic organ and buffers the functions of the liver |
Studies in Asian populations showed that sarcopenia is associated with the presence and severity of MASLD |
|
Disrupted adipose–liver–muscle axis in MASLD due to dysfunctional myokines74 |
Prevalence of sarcopenia in subjects without MASLD, with NAFL, and with MASH were 8.7%, 17.9%, and 35.0% |
|
|
Ectopic fat storage in muscles causing IR |
Skeletal muscle steatosis increased significantly with increasing stage of MASH |
|
|
Metabolic stress on Liver |
Lipoapoptosis is a principal feature of MASH that results from the failure of hepatocytes to dispose of excess FFAs.74 |
Biopsies of patients with MASH show decreased activity of FADS1 genes (encoding Liver Delta-6D and Delta-5D activities[1]) which is a key player in accumulating toxic lipids during MASH progression.86 |
Table 3 Risk Factors for MASH
ADIPOQ gene, Adiponectin; C1Q And Collagen Domain Containing, AHR, aryl hydrocarbon receptor; CIH, Chronic Intermittent Hypoxia; FADS1, fatty acid desaturase 1; FFA, free fatty acid; MRS, magnetic resonance spectroscopy; MASLD nonalcoholic fatty liver disease; MASH, non-alcoholic steatohepatitis; OSA, Obstructive Sleep Apnoea; PCOS, polycystic ovarian syndrome; LEP gene, leptin gene; TGF-B; transforming growth factor beta.
There is a concept of metabolic inflexibility which leads to deranged of metabolic control in the body which leads to development of MASLD and MASH. The liver is incapable to shift back and forth between prandial and fasting states compliantly because of exacerbated insulin resistance which is an indication of MASLD/MASH.64 Metabolic inflexibility is associated with hyperinsulinemia and systemic lipotoxic cell stress resulting in inflammation and fibrogenesis, and ultimately MASH. Metabolic inflexibility tends to be higher in patients with diabetes mellitus and obesity.
Insulin resistance has been reported with patients with Lean MASH associated with metabolic syndrome. In a North Indian study, out of 117 patients with insulin resistance, twenty-three (15.3%) patients were lean NAFLD with BMI 21.6±1.5, WC 82.9±4.7 (BMI < 23, WC < 90 cm in men and < 80 cm in women) 80% of these 23 (18/23) had insulin resistance with mean HOMA-IR of 3.4±.1.9. Only 4 (17%) did not have any component of metabolic syndrome
Diagnostic challenges & unmet needs of MASH
MASH needs to be differentiated from simple steatosis as it has prognostic importance. Liver function tests like measuring plasma liver aminotransferase levels are unreliable and do not confidently predict significant hepatic fibrosis. Figure 1 with the increasing need for liver transplantation, it is critical to accurately recognize and diagnose MASH in those with MASLD and its risk factors, as these patients are more prone to develop accelerated progression.6-9 Imaging techniques and biomarker panels have been evolving for the diagnosis of MASH/MASLD. The features and challenges of these methods are depicted in Table 4-6.
|
Modality |
Features |
Challenges |
|
US |
US Widespread availability Inexpensive compared to other techniques |
Cannot differentiate necro-inflammation and fibrosis from simple steatosis, so cannot be used for the evaluation of steatohepatitis Limited use in obese and pre-existing liver diseases Low sensitivity if liver fat is <30% Subjective interpretation-highly operator dependent Not routinely recommended92 |
|
CT |
CT Widespread availability |
Cannot differentiate necro-inflammation and fibrosis from simple steatosis, so cannot be used for the evaluation of steatohepatitis Potential radiation exposure Expensive |
|
MRI |
MRI Evaluation of the whole liver Accurate for steatosis |
Cannot differentiate necro-inflammation and fibrosis from simple steatosis, so cannot be used for the evaluation of steatohepatitis Expensive Susceptible to biases (T1 bias, T2 decay) |
|
TE-VCTE |
TE-VCTE Good predictive performance to predict cirrhosis in lean patients Can reliably differentiate various stages of fibrosis Useful and cost-effective in MASH Can be performed at the point of care Takes minutes to perform the test- results are instantaneous No sedation is required Preferred over the US as it can quantify liver fat and fibrosis for risk stratification during the same testing |
No precise cut-off levels for different stages of fibrosis Difficult to perform in obese patients due to reduction in transmitted vibrations by fatty tissue Region of interest (ROI) is smaller compared to MRE (1 cm X 4 cm vs entire liver 10.5 cm X16 cm) |
|
ARFI |
ARFI Similar diagnostic efficacy with TE Provides both a qualitative measure of displacement and a quantitative measure of SWV |
Difficult to perform in obese patients Measurements can be complicated by steatosis and hepatic inflammation |
|
|
MRE Highly accurate for detecting liver fibrosis because it can accurately quantify lipid fraction relative to water in tissue and can be used for the assessment of fat content Can analyse the entire liver |
Expensive Limited availability Does not replace the gold-standard ‘biopsy’ for MASH diagnosis |
Table 4 Features and Challenges with Current Imaging Modalities9
ARFI, Acoustic radiation force impulse; CT, Computed tomography; MRE, Magnetic resonance elastography MRI, Magnetic resonance imaging; SWV, Shear wave velocity, TE, Transient elastography; US, Ultrasound; VCTE, vibration-controlled transient elastography.
|
Marker |
Mechanism |
Features |
Limitations |
|
HA[1] |
Serum levels are dependent on production, increase with increased collagen synthesis, as well as degradation, which occurs in liver sinusoidal endothelial cells
|
Correlates well with hepatic fibrosis AUROC: 0.89 for advanced fibrosis |
Levels change according to the fasting status of patients Displays large intraindividual variations in both health and CLD
|
|
Fuc-Hpt[1] |
Glycoprotein is secreted into bile, but not into sera in the normal liver while these increase MASH (ballooning hepatocytes) |
Distinguish MASH from simple steatosis AUROC: 0.73 of biopsy-proven MASH 0.72 for detection of advanced fibrosis |
- |
|
Mac-2 binding protein (Mac-2bp). |
Undetectable in normal liver but is easily detected in MASH patients due to a significant increase in It closely correlates with the fibrosis severity and hepatocyte ballooning |
AUROC 0.81 for MASH |
- |
|
Fuc-Hpt–Mac-2bp combination |
|
AUROC: 0.85 |
-
|
Table 5 Features and limitations of Biochemical Markers
AUROC, area under Receiver operating characteristic; CLD, chronic liver disease, MASH, non-alcoholic steatohepatitis; Fuc, Hpt Fucosylated haptoglobin; HA, hyaluronic acid.
|
Modality |
Combination of |
Utility |
Limitations |
|
FibroTest |
Age, gender, bilirubin, GGT, ALP-A1, haptoglobulin, α2-MG |
AUROC: 0.84 for advanced fibrosis |
Cannot show different degrees of fibrosis Failure in Gilbert syndrome, cholestasis, and acute inflammation For distinguishing minimal fibrosis from intermediate fibrosis, it falls short (AUROC for F1 vs F0: 0.53) and a liver biopsy is still needed for definitive staging
|
|
ELF |
TIMP1, HA, P3NP, BMI, DM/IFG, AST/ALT, platelets, albumin |
Has a high sensitivity & specificity May show different stages of fibrosis Can predict liver-related clinical outcomes AUROC: 0.98 for advanced, 0.93 for moderate fibrosis |
- |
|
Hepascore |
HA, α2-MG, bilirubin, GGT, age, gender |
Highly accurate in detecting advanced fibrosis AUROC: 0.90 for cirrhosis |
Elevated liver enzymes with unknown etiology, reduce the predictive ability |
|
MASLD Fibrosis Score |
Age, BMI, IFG/T2DM, AST, ALT, platelet, albumin |
Most extensively validated system May show different stages of fibrosis AUROC: 0.88 for advanced fibrosis Can avoid liver biopsy in 3 of 4 MASLD patients with suspected fibrosis |
Scores between two cut-off values are common |
|
Fibrosis-4 (FIB-4 ) |
Age, ALT, AST, platelets |
Easy, simple, and inexpensive with quick results AUROC: 0.85–0.87 for advanced fibrosis More useful in moderate to advanced fibrosis cases where it can reduce the number of liver biopsies |
False-negative results Young age and normal platelet count may cause FIB-4 failure Cannot distinguish between simple steatosis and MASH
|
|
Fatty liver Index |
BMI, waist circumference, triglyceride, GGT |
Correlates well with US images in steatosis AUROC: 0.84 for detecting fatty liver |
Cannot show the presence of MASH or fibrosis |
|
Index of MASH |
Waist-to-hip ratio, triglyceride, ALT, HOMA |
AUROC: 0.88 for steatohepatitis A score of ≥50 has 92% specificity for MASH |
Needs to be externally validated |
|
BARD |
BMI, AST, ALT, T2DM |
Easy to measure AUROC: 0.80 for advanced fibrosis (0.88 with the addition of INR to measurement) |
Sensitivity and specificity are lower than in other panels High false positivity |
|
AAR |
AST, ALT |
Easy to measure |
High false positivity in alcohol users |
|
APRI |
AST, platelets |
Easy and cheap to measure Can exclude significant fibrosis AUROC: 0.76 for advanced fibrosis |
Cannot show different degrees of fibrosis |
Table 6 Features and Limitations of Biomarker Panels
AAR, AST-ALT ratio; AST, Aspartate transaminase; ALT, Alanine transaminase; Area Under the Receiver Operating Characteristics, α2-MG, Alpha 2 macroglobulin; ALP-A1, Apolipoprotein A1; BMI, Body mass index; ELF, enhanced liver fibrosis test; GGT, Gamma glutamyl-transferase; HA, Hyaluronic acid; IRI, Immunoreactive insulin; IFG-impaired fasting glucose; MASH-non-alcoholic steatohepatitis; P3NP, Aminoterminal peptide of pro-collagen 3; T2DM, type 2 diabetes mellitus; TIMP1, Tissue inhibitor matrix metalloproteinase 1.
Multiple biomarkers which incorporate clinical, biochemical and imaging markers can help to identify significant fibrosis (Table 6). Panels for the diagnosis of fibrosis have good specificity and negative predictive value (NPV) that allow the clinician to rule out advanced fibrosis, but they lack adequate sensitivity and positive predictive value (PPV) to establish the presence of advanced fibrosis. Therefore, several individuals fall in the “indeterminate-risk” group who need to be further evaluated with ELF test. All the clinical panels work better as patients with advanced disease have better prediction with marker panels.
The gold standard for diagnosis of MASH is Liver biopsy which is traditionally done via percutaneous route using ultrasonographic guidance. Endoscopic ultrasound (EUS) guided liver biopsy is now an established procedure that offers an alternative to conventional percutaneous and transjugular liver biopsy. EUS guided liver biopsy is seemingly is equally safe and better pain scores post biopsy. The safety and efficacy of EUS liver biopsy and confirming histopathological diagnosis has been evaluated in various studies.65,66 Biopsy however. It is invasive and associated with potential adverse effects, such as pain, bleeding, and infection and has poor acceptability. It requires significant expertise, has intra-observer and interobserver variability, and possibility of sampling variability. Considering these factors along with the fact that it least impacts the choice of treatment alternatives in clinical practice settings, biopsy either by traditional or endoscopic method are impractical to use in large populations. At best, it may help in identifying patients with autoimmune hepatitis when the diagnosis is in doubt. It is primarily limited to research for assessment of clinical endpoints in pharmacological studies. Alternatively, though not validated, non-invasive tests both blood tests and imaging studies are used for the diagnosis of MASH and serve the purpose for choice of appropriate management.67
Another important factor leading to deviation from guideline-practice is lack of health insurance coverage. Lack of health insurance coverage and inadequate coverage are important reasons for high out-of-pocket health expenditures. It typically forces people to delay or postpone medical care. The doctor’s reluctance to advise the patients to undergo various diagnostic tests due to out-of-pocket expenditure. Thus, resource constraints result in avoidance of recommended tests and frequency of follow up tests to control the expenditure. This is specifically an important factor in countries like India where out-of-pocket expenses accounts for about 62.6% of total health expenditure - one of the highest in the world as per a recent study.68
Cardio-metabolic associations of MASLD and MASH
It has been clear over the years of the close association between metabolic syndrome and MASLD/MASH with various meta-analysis showing that almost 70% of patients with MASH had metabolic syndromes. Having MASLD is now considered as an independent risk factor for development of a coronary event. The accumulation of visceral adipose tissue (VAT) that usually accompanies MASLD has been theorized to be responsible, in part to the extra hepatic co-morbid conditions, with the increased release of free fatty acids (FFA) in the circulation leading to development of hepatic insulin resistance and hepatosteatosis. This along with alterations in the hepatic lipid metabolism leads to development of atherogenic dyslipidemia, especially elevated plasma triglyceride concentrations and small dense LDL particles which develop atherosclerotic plaques. Elevated levels of Triglyceride rich lipoproteins (TRLs), particularly ones containing apolipoprotein B (ApoB) are hallmark of MASLD and represent a biochemical markers for elevated CVD risk. Furthermore, altered glucose metabolism and insulin resistance, exacerbate cardiovascular metabolic risk in these patients. Recently, Proprotein convertase subtilisin/kexin type 9 (PCSK9) has been linked to severity of hepatic steatosis and its gain of function mutation is associated with hypercholesteremia. This leads higher morbidity and mortality associated with cardiovascular diseases in patients suffering with MASLD or MASH. There are also some unanswered questions, the most important one being whether the association is due to shared risk factors or there is a role of hepatic steatosis in overall development of atherosclerosis. In addition, it still remains difficult to identify patients in this cohort, as which patients with MASLD will develop severe CVD complications or severe liver complications.
Non-pharmacological, Pharmacological, and Surgical Alternatives for the Treatment of MASH
Interventions for Weight Loss
Non-pharmacological
Weight loss is one of the strongest pillars for management of MASH patients, which improves histology along with the cardio-metabolic profile and glucose homeostasis.69,70 Significant data suggests tells us that 5-10% weight loss results in an improvement in steatohepatitis in 58-90% patients with MASH.71 Significant improvement (40%) have also been reported in Asian patient population with 3–5% weight reduction.72,73 In patients with obesity and MASH, ≥ 10% weight loss has also shown significant regression of hepatic fibrosis.74 Not only obese but lean patients with MASH can also benefit from similar dietary and weight management strategies. A 12-month study comparing lean and non-lean patients found that any amount of weight reduction was associated with significantly improved steatosis, ballooning, and NAS scores in both groups.75 These data support the notion that weight loss in terms of loss of adiposity improves histology in patients with MASH regardless of baseline BMI.76
Exercise has demonstrated benefits beyond weight loss in patients with MASH with its duration being proportional to improvement of hepatic steatosis.77 There is a growing evidence which shows advantage of one type of exercise(aerobic, resistance, high intensity, or low-intensity exercise) over others but it is currently inconsistent in various studies. Recommended frequency for exercise is more than 30 minutes in a day for 5 days a week.78
A comparison of low calorie diet and aerobic exercise with low calorie diet alone in a short term study showed that significant improvement in blood pressure, FPG, TG, homeostasis model assessment-estimated insulin resistance, US grading of steatosis, and Quality of life only in patients with MASH who followed aerobic exercise.79 Maintenance of the weight loss is challenging due to its association with changes in dietary habits, and lifestyle.102 This necessitates a diet plan and exercise type based on patients’ preferences to ensure long-term adherence.
Thus it is clear from the above data, that combination of a dedicated exercise regimen and calorie restricted diet has synergistic effect which leads to improvement in hepatic steatosis and inflammation103-104 (Figure 3).
Pharmacological and surgical interventions
Diet and exercise alone are usually helpful in initial weight loss but have been found limited in maintenance of weight loss.105 Weight loss and its maintenance are challenging consequently a larger patient pool may need alternate methods to restrict excessive calories.80 These include patients unable to achieve >5% loss of total body weight with lifestyle interventions or unable to sustain, or who have a BMI ≥27 kg/m2 along with ≥1 metabolic comorbidity, or those with a BMI >30 kg/m2 with comorbidities. Over the years, several pharmacological agents which can achieve weight loss through different mechanisms have been tried in these patient groups.106,107 However, among the plethora of approved medications only one glucagon-like peptide-1 (GLP-1) agonist, has been found to improve liver histology in MASH patients in a phase II study.81 Sodium-glucose cotransporter-2 (SGLT2) inhibitors have also been shown to be effective for weight loss and reduced hepatic fat content and may thus be beneficial in MASH.82 However, the need for chronic use, monitoring, and associated adverse events and sparse clinical evidence for improvement of MASH parameters limit the utility of these medications. Therefore, preference should be given to lifestyle interventions.
Bariatric surgery has also been studied for weight loss and benefits extrapolated in patients with MASH.108,109,110 The largest prospective study done initially demonstrated a likely deterioration of fibrosis, with an increase in the severity of fibrosis in ~20% of patients during a one-year follow-up period, possibly due to rapidity of weight loss.83 However, a study in severely obese patients with biopsy-proven MASH found improvement of MASH in 84% of patients with progressive and sustained reduction of fibrosis that began during the first year and was sustained through 5 years.84 The intended benefits of the bariatric surgery should be seen along with the risks of weight loss surgery particularly when indication is just for MASLD and MASH85 Further up to 20% of patients have normal BMI, in these patients for less severe obesity and related complications, including T2DM, endoscopic bariatric metabolic therapies86 (EBMT) are rapidly emerging as less invasive therapies These include intragastric balloons (IGB), endoscopic sleeve gastroplasty (ESG), and duodenal mucosal resurfacing (DMR).111, 112 IGBs and ESG has shown to achieve adequate and sustained weight loss required for MASH improvement without significant adverse events.87 These EBMTs can bridge the gap between lifestyle interventions having high safety but limited efficacy, while bariatric surgery has high efficacy with poor safety profile.88 The last resort is Liver Transplantation indicated by practice guidelines for patients with MASH and ESLD or HCC only.113,114 But a study has shown that within a month of LT, 40% of patients with MASH were identified to be at risk of developing renal dysfunction implying serious safety issues in this patient population. An alternative investigated in patients predominantly suffering from MASH (>50% MASH Cirrhosis) includes sleeve gastrectomy during LT which maintained greater weight loss and had fewer components of the metabolic syndrome.89 Thus, a reduction in risk factors for post-LT metabolic syndrome may confer a significant survival benefit.
Current drug therapies targeting downstream and upstream pathways
Pioglitazone demonstrated improved hepatic steatosis, ballooning necrosis, inflammation, and fibrosis in steatohepatitis patients with or without prediabetes or T2DM.90-93 However, these beneficial effects of pioglitazone are effected by an increased risk of weight gain, significant edema, rare possibility of development of bladder cancer, and a decrease in bone mineral density.115-119 Some clinical trials did demonstrate significant reductions in steatosis, lobular inflammation and fibrosis in patients treated with vitamin E (400 IU).95-97 However, it was associated with an increased risk of all-cause mortality linked to hemorrhagic stroke and prostate cancer.98,120
In a recent Phase 3, randomized, controlled trial, Harrison et al showed that a newer oral, liver directed, thyroid hormone receptor beta selective agonist, Resmetirom was studied in 966 patients with MASH and different stages of fibrosis. The oral drug improved the MASLD activity score by 2 points with no worsening of fibrosis. There was also improvement in fibrosis by one stage with no worsening of activity scores. This lead to its approval for patients with fibrosis due to MASH by FDA. This is the first drug to be approved for this indication.
With better understanding of the MASH pathophysiological pathways and identification of newer target pathways, various drug classes are being evaluated for their efficacy. Majorly the drug classes target either metabolic pathways, fibrosis or oxidative stress. The drug classes acting on the metabolic pathways have demonstrated some clinical benefit due to inhibition of de novo lipogenesis, improved insulin sensitivity, rectification of the link between de novo lipogenesis and bile acid metabolism, increased β-oxidation of fatty acids in the mitochondria and modulation of the uptake of fatty acids in the liver via thyroid hormone receptors.99-101 The drugs are currently in phase IIb and phase III trials and their detailed description has been depicted in Table 7.102 The clinical outcomes of most of the remaining investigational drug classes have been disappointing.
|
Drug(s) |
Phase |
Clinical outcome |
|
Metabolic Pathways |
||
|
|
||
|
1. Liver-targeted Acetyl-coenzyme A carboxylase (ACC) inhibitor and diacylglycerol acyltransferase 2 (DGAT2): Inhibition ACC is the first committed enzyme in the hepatic de-novo Lipogenesis (DNL) pathway. DGAT2 is highly expressed in the liver and adipose tissue and catalyses the terminal step of DNL, specifically the esterification of a fatty acid with diacylglycerol to form triglyceride. Independent inhibition of each of these steps has been shown to reduce hepatic steatosis. |
||
|
Ervogastat (DGAT2i) + Clesacostat (ACCi) |
IIb |
Dose-dependent reductions in both liver fat and serum triglycerides Direct antifibrotic effects in hepatic stellate cells, the collagen-producing fibroblast population in the liver DGAT2i+ACCi reduced steatosis as well as inflammation and fibrosis markers without the expected ACCi-associated increases in serum triglycerides. Phase IIa trial (6-wk) in patients with MASLD, Combination reduced hepatic steatosis to a greater degree than DGAT2i alone, as assessed by magnetic resonance imaging-proton density fat fraction Metabolic Interventions to Resolve MASH with fibrosis-68week study |
|
2. FXR agonist: FXR nuclear receptors are expressed in the liver and intestines. Activation reduces bile acid synthesis and uptake of bile acids in the ileum by downregulating the sodium-dependent bile acid transporter. Regulates cholesterol lipoprotein and bile acid metabolism to modulate immuno-inflammatory and fibrogenic responses |
||
|
Obeticholic acid |
III |
FLINT: Improved the histological features No significant resolution of MASH REGENERATE: improvement in fibrosis by 23% |
|
Tropifexor |
IIb |
FLIGHTFXR: Recruitment TANDEM: Recruitment |
|
3. PPAR-α/δ agonist: regulates lipid and insulin metabolism |
||
|
Seladelpar |
IIb |
Phase 3, discontinued |
|
Lanifibranor |
IIb |
Awaiting Phase 3 NATIVE: Resolution of MASH and Fibrosis without worsening of either |
|
Elafibranor |
III |
GOLDEN: MASH was resolved without the worsening of fibrosis in 19% RESOLVE-IT: No histological benefit, discontinued |
|
Saroglitazar |
Approved by DCGI, India (not USFDA) |
significant improvement in transaminases, LSM, CAP, glycemic control, and lipid parameters |
|
4. THR-ß agonist: (THR β) is highly expressed in hepatocytes and is responsible for regulating the metabolic pathways in the liver that are frequently impaired in MASLD and MASH |
||
|
Resmetirom |
Approved by USFDA(March 2024) in conjunction with diet and exercise in non cirrhotic patients with significant fibrosis |
Relative decrease in liver fat Significant improvement in steatohepatitis Significant reductions in ALT and AST levels, atherogenic lipids, lipo-protein(a), markers of inflammation and fibrosis as well as improvement in MASH on liver biopsies |
|
5. FGF analogues: regulates bile acid synthesis, glucose homoeostasis and energy homoeostasis |
||
|
Pegbelfermin |
IIb |
Reduction in liver fat content with an acceptable safety profile |
|
NGM282 |
IIb |
|
|
6. MPC inhibitor: insulin sensitizer that has been shown in initial studies to increase lipid oxidation and reduce de novo lipid synthesis and gluconeogenesis in the liver |
||
|
MSDC-0602K |
IIb |
Phase 2/3 No Significant effects |
|
7. GLP-1-GIP co-agonist |
||
|
Tirzepatide |
III
Phase II Results published (SYNERGY NASH Study in 2024) |
Improves glucose disposal and also reduces nausea associated with GLP-1 activity Significant weight loss in obese individuals 51.8%, 62.8% and 73.3% of participants taking 5 mg, 10 mg and 15 mg, respectively, achieved an absence of MASH with no worsening of fibrosis on liver histology compared to 13.2% of participants on placebo at 52 weeks of treatment |
|
8. GLP-1-glucagon agonist: Glucagon activates lipid oxidation and also improves mitochondrial turnover and function |
||
|
Cotadutide |
IIb |
- |
|
Semaglutide |
Phase II results published |
Significantly higher percentage of patients with MASH resolution than placebo No significant improvement in fibrosis stage versus placebo |
|
AntiFibrotic |
||
|
1. Lysyl oxidase-like 2 inhibitor: blocks the formation of collagen bands, resulting in an anti-fibrotic effect. |
||
|
Simtuzumab |
II |
No robust findings yet |
|
2. Galectin-3 inhibitor: target both secreted and membrane-associated galectins reduces liver fibrosis and portal hypertension |
||
|
Belapectin |
III |
NAVIGATE Recruiting phase |
|
Oxidative stress |
||
|
1. CCR2/CCR5 inhibitor |
|
|
|
Cenicriviroc |
III |
Terminated |
|
2. SCD1 inhibitor: inhibiting de novo fat synthesis |
||
|
Aramchol |
III |
ARRIVE: No benefit ARREST: a decrease in steatosis and inflammation and ballooning along with a modest improvement in fibrosis |
|
3. ASK1 inhibitor III: |
||
|
Selonsertib |
III |
STELLAR trial: Discontinued |
|
4. Caspase inhibitor: pan-caspase inhibitor that blocks liver cell-related apoptosis and inflammatory responses |
||
|
Emricasan |
II |
ENCORE trials: Discontinued |
Table 7 Investigational Drugs for The Treatment of MASH
ARRIVE, aramchol for HIV-associated MASLD and lipodystrophy (ARRIVE) trial; ARREST, Aramchol for the REsolution of Steatohepatitis; ASK-1, apoptosis signal-regulating kinase 1, CCR2/CCR5- dual chemokine receptor, Acetyl-coenzyme A carboxylase (ACC) inhibitor, (DGAT2) diacylglycerol acyltransferase 2, ENCORE- Emricasan, an Oral Caspase Inhibitor, in Subjects With MASH Cirrhosis and Severe Portal Hypertension; FLIGHT-FXR- Study of Safety and Efficacy of Tropifexor (LJN452) in Patients With Metabolic dysfunction associated steatohepatitis(MASH); farsenoid-x-receptor; FLINT-Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis; TANDEM- Study of Safety, Tolerability, and Efficacy of a Combination Treatment of LJN452 and CVC in Adult Patients With MASH and Liver Fibrosis; NATIVE- MASH to Assess IVA337; SCD1- Stearoyl-coenzyme A desaturase 1, THR-thyroid hormone receptor; RESOLVE-IT- Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients With Nonalcoholic Steatohepatitis; STELLAR- Safety and Efficacy of Selonsertib in Adults With Nonalcoholic Steatohepatitis (MASH) and Bridging (F3) Fibrosis.
Being one of the commonest diseases effecting the metabolic organ of the body, MASLD and MASH require a thorough management and newer insights into its pathogenesis. Though liver biopsy is the possibly the only validated method of confirmation of diagnosis there has been progress in development of alternative biomarkers and imaging modalities. These methods seem to have provided improved understanding of the pathological changes in structure and function of the liver in recent years. Weight loss through lifestyle intervention remains the cornerstone of MASH therapy, the adherence to which is truly challenging and subjective. Though bariatric surgery shows significant advantages in terms of decline of fibrosis, patients with advanced disease are not appropriate candidates for it. Lean MASH patients have shown to develop more severe disease than obese patients, for whom pharmacotherapy is the only alternative. Few existing treatment alternatives include use of therapies like SGLT2i or antioxidants, vitamin E which have shown some benefits in patients with MASH. But there is a need for larger randomized trials with these drugs specifically in patients with MASH demonstrating histological benefits, attenuating progression to fibrosis and HCC. Newer drug classes targeting different sites are being investigated. Most agents are in phase 2 or 3 of their developmental phase. Currently, though results with certain classes like CCR2/CCR5 inhibitor, SCD1 inhibitor, ASK1 inhibitor III and Caspase inhibitor have been disappointing few have shown encouraging results. These include GLP-1-GIP co-agonist, FGF analogues, THR-ß agonist, few PPAR-α/δ agonist, FXR agonist and DGAT2i/ACCi. These agents have shown reduction in hepatic fat content, attenuation of fibrosis and a good tolerability profile in phase II studies. The results of larger phase 3 studies with these agents will end the long wait for an effective and well-tolerated universally approved drug class for the treatment of MASH.
MASLD and MASH are driven by the obesity and diabetes epidemic, poor lifestyle, compounded by dysbiosis and influenced by genetic factors in others. These aspects should become a new priority given the poor outcomes with associated hepatic and extrahepatic events. Though not completely successful, the developments in the management of MASH have been fairly encouraging. Further well-designed long-term prospective studies should be undertaken to generate evidence with definitive results.

©2024 Choudhuri, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.