eISSN: 2373-6372


Case Report Volume 15 Issue 4
1Department of Radiology, Dorot, Medical Center for Rehabilitation and Geriatrics, Israel
2Department of Rehabilitation, Dorot. Medical Center for Rehabilitation and Geriatrics, Israel
Correspondence: Michael D Levin, Department of Radiology, Dorot, Medical Center for rehabilitation and geriatrics, Netanya, Israel
Received: November 13, 2024 | Published: November 20, 2024
Citation: Levin MD, Mendelson G. Late diagnosis of congenital anal canal stenosis. Case report and literature analysis. Gastroenterol Hepatol Open Access. 2024;15(4):126-129. DOI: 10.15406/ghoa.2024.15.00589
We describe a case of congenital stenosis in the anal canal (ACS) in an elderly woman who suffered from severe constipation since early childhood. Digital examination revealed a thin membrane in the anal canal with an opening through which only the index finger could fit. An X-ray examination revealed a narrowing in the anal canal at 1.9 cm from the normally formed anus. The Foley catheter balloon was stuck above the membrane. We found only one article describing delayed diagnosis of congenital rectal stenosis in a 17-year-old adolescent. But she also had a membrane in her anal canal. A PubMed search identified 14 articles that published radiographic studies of rectal atresia and rectal stenosis in children. In all cases radiometric analysis revealed atresia or stenosis not in the rectum, but in the anal canal. In 8 cases it was possible to determine the exact location of the membrane. It was located approximately 1 cm from the anal verge. Conclusion. An analysis of articles proved that in all cases the membrane was not in the rectum, but in the anal canal. This discovery allows the membrane to be excised through the anal approach without damaging the anal canal and the innervation of the anorectal area. It has been shown that in a significant number (≈5%) of children with functional megacolon, ACS may be the cause of the pathology. Scientifically based methods for diagnosing and treating ACA/ACS are proposed. The localization and form of the defect suggests that it occurs in the embryonic period due to a violation of the rupture of the membrane in embryos 13.5–135 cm long.
Keywords: rectal atresia; rectal stenosis; anal canal atresia; anal canal stenosis; anorectal malformations; embryology.
RA, rectal atresia; RS, rectal stenosis; ACA, anal canal atresia; ACS, anal canal stenosis; CAS, congenital anal stenosis; ARM, anorectal malformations; IAS, internal anal sphincter; EAS, external anal sphincter; PRM, puborectalis muscle.
Chronic constipation is widespread. It is believed that between 10% and 15% of the population are treated for constipation on an ongoing basis. The Rome IV criteria classify chronic constipation disorders into four subtypes: (a) functional constipation, (b) irritable bowel syndrome with constipation, (c) opioid-induced constipation, and (d) functional defecation disorders, including inappropriate bowel movements and dyssynergia. Defecation.1 Chronic constipation also occurs with anal stenosis. Several classifications of anal stenosis have been proposed, but the Khubchandani classification is considered the most successful.2 He divides stenoses into primary and secondary. Primary ones include congenital stenoses, as an element of anorectal anomalies. These also include senile or involutional stenoses, the nature of which is not entirely clear. Secondary stenoses include postoperative stenoses: after hemorrhoidectomy, removal of perineal masses, fistulectomy, sphincteroplasty, papillomas fulguration or tumor removal.2 Congenital anorectal stenoses include congenital anal stenosis (CAS) and rectal stenosis (atresia).3 Since the prevalence of anorectal malformations is 3.5 per 10,000 live births (95% CI 3.4–3.6),4 and SPC occurs in 0.7% of them,3 the number of newborns with SPC is 2 per 1 million newborns. However, these cases are reported in the first days or weeks after birth, when the opening is very narrow, preventing defecation. In some cases, when the opening is wider, severe constipation occurs only after a few months, when unformed stools are replaced by formed ones. If the opening is wide enough, then constipation may occur in the long term, when the diameter of the stool increases with age, while the diameter of the rigid opening of the stenosis does not change with age. We present a case of congenital anal stenosis in an elderly woman suffering from severe constipation since childhood.
A 78-year-old woman permanently resides in a closed department due to schizophrenia. Severe constipation appeared at early school age. All her life she was helped only by cleansing enemas and digital removal of feces. Defecation was always painless. With age, problems with defecation increased so much that the patient began to have suicidal thoughts. Recently she has been taking Bisacodyl tablets 10 mg 2 times a day and Lactulose solution 30 ml 2 times a day. She has constant unformed, but not liquid, stools, which she does not control. Upon examination, a large belly is noticeable. A digital rectal examination performed because of prolonged defecation retention revealed a narrow, hard, thin-walled ring in the anal canal a few centimeters from the anus. The hole in the ring made it possible to insert an index finger about 2 cm wide into the rectum. A large, very dense fecal stone was felt above the membrane, which, during manual examination, was broken into fragments and removed.
X-ray examination and radiometric analysis of radiographs
By measuring any distance of known length on a radiograph, other parameters on that radiograph can be calculated. To do this, the projection distortion coefficient is calculated. For example, it is known that the true length of the anal canal in adults is about 4 cm. The ratio of the true length of the anal canal (4 cm) to its length on the radiograph is the coefficient of projection distortion. To find out the other true dimensions on this radiograph, their length should be multiplied by the coefficient of projection distortion.
In our case, the perineum in front and behind the anus was smeared with barium paste to determine the location of the anus as the point of intersection of the enema tip with the contrast line on the perineum. 300 ml of barium suspension was injected into the rectum (Figure 1a). After removing the enema tip, a No. 20 Foley catheter was inserted into the rectum without any effort. The balloon was inflated to a diameter of ≈ 2.5 cm and pulled into the anal canal as far as it would go. The balloon stopped over the constriction at 1.9 cm from the anus (Figure 1b).
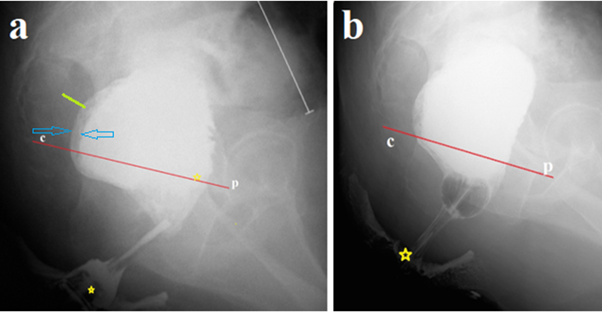
Figure 1 The study was carried out after colon cleansing. (a). A lateral radiograph of the anorectum was taken after administration of 300 ml of barium. It is known that the true length of the anal canal between the anus and the pubococcygeal line (p-c), i.e., between two yellow stars, is 4 cm. The tone of the rectum is increased, as evidenced by the expansion of the retrorectal space (green line - 0 .8 cm) and barium in the folds of the mucosa between the blue arrows. The presence of barium below the p-c line indicates insufficiency of the puborectalis muscle (descending perineal syndrome). The sigmoid colon (white line) is significantly dilated to 4 cm (b). The Foley catheter balloon, inflated in the rectum to a diameter of ≈ 2.5 cm, became lodged in the anal canal above the stenosis, 1.9 cm from the anus.
The diagnosis of congenital membranous stenosis of the anal canal. The patient refused further examination and treatment.
We found in the literature only one case of late diagnosis of anal stenosis,8 which the authors, in accordance with accepted ideas, call rectal stenosis.
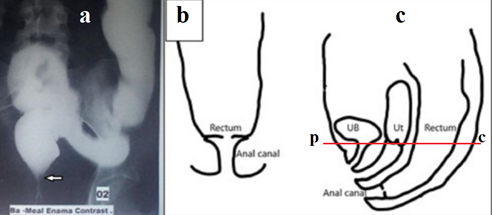
Figure 2 Figures and description from article by Chowdhury et al.8 In 17‐year‐old girl meconium passed within 24 h of life. She developed features of constipation after weaning at about 6 months of age. Gradually the constipation worsened. She did not pass stool for the last 9 days at the time of referral. Barium enema showed a dilated colon, and the lower end of the rectum was located high up with a beak‐like appearance (Figure 1a). Digital rectal examination under anesthesia revealed a thick diaphragm, about 3 cm from anal verge with a central hole, through which hard fecal matter was visible (Figure 1b&c). The thick membrane was excised circumferentially using monopolar coagulating diathermy from anal approach.
The sigmoid and descending colon are dilated, but the sigmoid colon is located in the small pelvis, that is, it is not elongated, which does not correspond to the severe chronic constipation described in the article. Second, the white arrow in Figure 1a shows the normal connection between the rectum and the closed anal canal. This means that the stenosis is located lower, i.e. in the anal canal. Thirdly, the diagrams do not correspond to the anatomical location of the organs. However, the membrane is located significantly below the pubococcygeal line we drew (red line). This line corresponds to the location of the puborectalis muscle, which is located between the rectum and the anal canal. From which it follows that the membrane is in the anal canal. Fourthly, the possibility of excision of the membrane from the anal access also indicates its location in the anal canal. Thus, the case described by the authors confirms our observation that the so-called rectal stenosis is in the anal canal, i.e., it is anal canal stenosis (ACS). Secondly, stenosis is a membrane with a hole in the center, which is also confirmed by a group study on the classification of anorectal anomalies in Japan.9
These results are of fundamental importance because: (1). The observations described above suggest that among patients with severe constipation, which according to clinical characteristics corresponds to functional constipation, there may be patients with SAC. (2). The possibility of using anal access to excise the membrane instead of wide dissections during pull-through operations, which lead to damage to the anal canal, impaired circulation and innervation of the rectum, has been shown; (3). Rectal atresia (RA) and rectal stenosis (RS) are always considered together since these types of anorectal malformations represent the same pathology. They differ only in the presence or absence of a central hole. To ensure the validity of these considerations, we searched PubMed for articles describing and illustrating the diagnosis of RA/RS.
In 6 newborns the atresia, and 2 infants had stenosis were in the anal canal and in these cases was possible to measure the approximate length of the anal canal above and below the level of obstruction. This was shown best in the article by Stenström et al.10 (Figure 3).
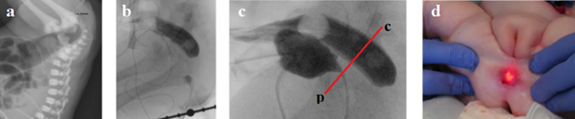
Figure 3 (a-b) At rest, the distance between the rectum, contrasted from the colostomy, and the distal segment, contrasted through the anus, was about 2 cm.10 (a). The black arrow shows the end of the rectal probe. (c). A distal colostogram under high pressure revealed disclosure of the upper part of the anal canal, distal to the pubococcygeal line we drew. (d). “The endoscope was pressed against the rectal atresia, and with the help of external pressure, the endoscope could be seen 1 cm up in the anal channel”.10
Atresia (6) and stenosis (2) was found in the anal canal in the form of a septum.10-17 The approximate dimensions of the upper and lower parts of the anal canal, i.e., above and below the septum, are given in Table 1.
|
№ Reference |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
|
above |
1 |
0.8 |
1 |
0.8 |
0.3 |
0.8 |
1.1 |
0.7 |
|
below |
0.7 |
0.9 |
3 |
0.8 |
1.4 |
0.9 |
1.2 |
1 |
Table 1 Approximate dimensions of the anal canal above and below the anal septum
All these observations are characterized by the presence of a septum below the pubococcygeal line, where the length of the anal canal above and below the septum could be measured. However, the figures in Table 1 are not accurate because no measuring instruments were used. In addition, more often only frontal radiographs were performed, on which it is impossible to accurately determine the size of the rectum and anal canal. The use of solid rectal catheters or Hegar dilators causes elevation of the pelvic floor and septum, which leads to a decrease in the length of the upper part of the anal canal.
In 8 cases, the rectum was located at the level of the p-c line, as is normal.17-22 This means that the atresia could only be located caudal to the rectum, i.e., in the anal canal. However, no evidence of either the presence of atresia itself or the location of the septum in the anal canal was found in the drawings in these articles. Thus, when analyzing 14 observations, not a single case was identified where intestinal obstruction could be localized in the rectum. We found only thin-walled membranes. The thickness of the membrane depended on the width of the anal canal. When the anal canal was dilated, the membrane was stretched, as in Figure 3, and looked thin. When the anal canal has been closed, as described by Chowdhury et al.,8 the membrane appears thick.
This study proves that the congenital pathology which commonly considered as rectal atresia or rectal stenosis (if there is an opening) is anal canal atresia (ACA) or anal canal stenosis (ACS). Anal canal stenosis should be distinguished from congenital anal stenosis (CAS), which is characterized by narrowing of the anus that located distal to the anal canal. ACA causes complete obstruction in newborns due to the presence of a membrane in the anal canal approximately 1 cm from the normally formed anus. Because of the misconception that atresia is in the rectum, far from the anus, pediatric surgeons perform a colostomy in the neonatal period and complete the correction with one of the pull-through operations that destroy the anal canal. If there is an orifice in the membrane (ACS), constipation occurs, the severity of which depends on the diameter of the orifice in the membrane. If the orifice is wide enough, such cases can pass under the mask of FM. For example, Clayden and Lawson, during anal dilatation, which was performed under general anesthesia by inserting up to 4 fingers of the operator’s hand, in 4 of 79 patients with FM they were unable to insert more than 2 fingers due to the presence of a rigid ring that was 1 cm from the anal verge.23 During histological examination of the IAS Duhamel found in some patient fibrous changes in the anal canal, which were like congenital anal stenosis.24 It follows from this that when examining patients with megacolon, one must consider the possibility of relatively wide anal stenoses, which cannot be determined by bougienage. Thus, anal distension, which is effective in treating FM, may also be useful for diagnosing secondary megacolon, i.e., ACS.
According to the study of Nobles (1984), at the junction of the endodermal and exodermal rudiments, the anal membrane at first appears, which ruptures during embryos with a length of 13.5–135 cm.25 Since the location of the membrane in anal canal atresia corresponds to the junction in the embryonic period of the endodermal and ectodermal rudiments of the IAS, it is likely that this pathology occurs because of disturbances of membrane ruptures during embryos with a length of 13.5– 135 cm.
Diagnosis in newborns with complete obstruction involves inserting a rigid catheter through the normally formed anus until it stops. In AAC, the catheter stops about 1 cm from the anal verge. 30 hours after birth, during fluoroscopy, a radiograph of the anorectum is taken in the lateral projection, at that moment when, during compression of the abdomen between the doctor’s palms, gas from the rectum penetrates the anal canal. The location of the membrane is determined between the gas that has penetrated the anal canal and the probe inserted through the anus. The reflex of the anal canal opening in ARM without a functioning fistula is shown in Figure 4.
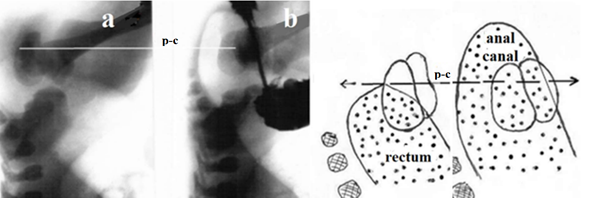
Figure 4 X-ray examination of a newborn with ARM without a visible fistula. (a). At rest, the gas was in the rectum at the level of the pubococcygeal line (p-c). (b). When the rectal pressure increased to a certain level, the anal canal opened, and the gas approached the skin of the perineum.
Treatment consists of excision of the membrane from the anal access, as described above.8,10 If, for technical reasons, the membrane is not accessible from the anal approach, the treatment technique described by us for the treatment of anorectal anomalies without a visible and non-functioning fistula can be applied.26 Reported cases of delayed presentation of congenital rectal stenosis suggest that they may be the cause of severe constipation, which is treated with a clinical diagnosis of functional constipation or functional megacolon. We identified a significant clinical effect after anal distension for functional megacolon in children,27,28 according to the method described in the article by Clayden and Lawson.23 Anal dilatation is performed under general anesthesia with alternate insertion of one to four fingers of the operator’s hand into the anal canal. Clayden and Lawson describe the following observation: - «Four of the 79 cases on anal dilatation were shown to have a minor degree of anal stenosis, with a string stricture at the mucocutaneous junction. This string stricture, approximately 1 cm from the anal verge, accepted only 2 fingers of the operator's hand during anal dilatation».23 These authors found typical signs of ACS in 5% of children with functional megacolon. Considering that anal dilatation has a pronounced therapeutic effect and does not damage the internal anal sphincter, like sphincterotomy, this method can be recommended both for the treatment of FM and for the timely detection of ACS.
For the first time, a case of congenital stenosis of the anal canal was described in an elderly woman who had suffered from severe chronic constipation all her life. An analysis of articles on rectal atresia and rectal stenosis in children, where radiographs were available that allowed for radiometric analysis, proved that the membrane was not in the rectum, but in the anal canal. It was located approximately 1 cm from the anal verge. This discovery allows the membrane to be excised through the anal approach without damaging the anal canal and the innervation of the anorectal area. It has been shown that in a significant number (≈5%) of children with a presumptive diagnosis of functional megacolon, the cause of the pathology may be ACS. Evidence-based methods for diagnosing and treating ACA/ACS have been proposed. The embryonic development of this type of anorectal malformations is probably associated with a violation of the breakdown of the membrane between the junction of the endodermal and exodermal primordia of the anal canal.

©2024 Levin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.