eISSN: 2373-6372


Review Article Volume 10 Issue 2
Department of Gastroenterology, Saint-Petersburg State Pediatric Medical University, Russia
Correspondence: Kornienko Elena Alexandrovna, PhD, Professor, Head of the Chair of Gastroenterology of the Department of Post-Graduate and Additional Professional Education of Saint-Petersburg State Pediatric Medical University, ul. Lytovskaya, 2, Saint-Petersburg, 194100, Russia, Tel +7-911-919-88-39
Received: January 23, 2019 | Published: March 5, 2019
Citation: Alexandrovna KE, Sultanovna KS. Intestinal microbiota in infants at the first half-year of life with functional disorders. Gastroenterol Hepatol Open Access. 2019;10(2):67–73. DOI: 10.15406/ghoa.2019.10.00358
Objective The aim of this study was assessment of fecal microbiota and the role of lactase deficiency (LD) in a group of infants with functional disorders (FD) and to evaluate whether Lactobacillus reuteri DSM 17938 improve these symptoms in a group of breastfed and formula fed infants.
Design Randomized open observational trial.
Setting State Pediatric Medical University in St. Petersburg ( Russia), Department of Gastroenterology
Participants 102 infants aged less than 3 months: 62 breastfed or formula fed infants meeting Rome criteria IV (treatment group): 23 with FD and LD, 39 with FD without LD received β-galactosidase or probiotic L.reuteri 17938 in drops or in infant formula. They were compared to 40 healthy infants (control group).
Interventions Oral daily L reuteri (1×108 colony forming units) in drops (19 breast fed FD infants) or in formula (20 formula fed FD infants) or 700 units of β-galactosidase (23 infants with LD).
Main outcomes measures The primary outcome was daily crying time, regurgitation frequency, stool frequency after 28 days of treatment. Secondary outcomes were calprotectin values, fecal microbiota composition (culture and real time PCR) after 28 days of treatment.
Results: LD has been revealed in 37% of FD infants. Infants with FD had higher calprotectin values and high level of pathobionts in fecal microbiome, than controls. Infants of treatment group, randomized according LD existence and type of feeding, showed a significant reduction in FD manifestation and calprotectin level, improvement of microbiota composition.
Conclusions: Admistration of L reuteri DSM 17938 as in drops, as in formula reduce FD manifestation as well as calprotectin values after 28 days of treatment sample of breastfed infants and formula fed infants with colic. These findings confirm the use of probiotics to treat colic in infants.
Keywords: intestinal microbiota, lactase deficiency, hydrogen breath test, functional disorders, infantile intestinal colic, calprotectin, L.reuteri
Functional disorders of the gastrointestinal tract play a significant role in early infancy. According to a recent meta-analysis performed by Y. Vandenplas et al.1 in 2015, the average prevalence of infantile regurgitations in early infancy is 26%, infantile colic– 21%, at that, functional constipations can be observed in 15% of infants.
Currently, pathophysiological mechanisms of FD development remain a partially unclear. Morphological and functional immaturity of gastrointestinal system and nervous regulation, transitory lactase deficiency, as well as peculiarities of intestinal microbiota formation could explain functional disorders in infancy.2
According to some author’s opinion, lactose intolerance due to immaturity of the intestinal epithelium and transitory lactase deficiency should be an alimentary cause of infantile colic together with immaturity of bile acid mechanisms that alter intraluminal and absorptive mechanisms, immaturity in motility and alterations in the microbiome3,4 During the first months of life, gut microbiota shows main modification. Its composition depends on several factors: mother’s health, type of delivery, breast feeding starting time, type of feeding, prescription of antibacterial drugs during pregnancy and delivery, duration of child’s stay at the maternity hospital.5 During the first months of life, the intestinal microbiota is relatively scanty, unstable, subjected to external and internal influences. In the process of its development, deviations of microbiome could influence gut function and motility. Many studies have proved that colicky infant’s gut microbiota shows a different pattern compare to non-colicky ones, with a significant increase of pathogenic bacteria such as E. coli.6–8
Further, we have to take in account that gut microbiota and local immune system are strongly linked. The intestinal microbiota exists in constant interaction with the immune system of the intestine. Under the influence of microbial stimuli, immune system cells are activated in order to regulate the differentiation of Th-lymphocytes, the production of cytokines and secretory IgA; permeability of the epithelium decreases and the protective barrier of the intestine strengthens. Morphologically, the process of colonization of the gut is accompanied by mild signs of inflammation in the mucous layer of the intestine. This mild inflammation reflects the process of adaptation and can be defined as “physiological”. The value of fecal calprotectin can be a suitable marker of gut inflammation. Calprotectin is an inflammatory protein produced by neutrophilic granulocyte during inflammatory process. The evaluation of fecal calprotectin in colicky and non-colicky infants carried out by JH Roads et al.9 showed that the values was increased in both groups (in the control group 197±46 µg/g while normal value in adults is lower than 50 µg/g), which indirectly suggest the presence of mild inflammation of intestinal mucosa. However, in subjects suffering from infantile colic, the values of calprotectin appeared to be higher than in the control group (413±71 µg/g, p=0.042).
Using PCR method, a higher rate of Klebsiella was found in colicky group infants, as well as a higher level of hydrogen in exhaled air. So, the authors concluded that an alteration of the intestinal microbiota and a related inflammatory pattern play an important role in the development of infantile colic, suggesting a connection between gut microbiota, pathological inflammation and motility disorders.9
The supposed link between inflammation and motility is based on the interaction of the intestinal immune system with the nervous one. Lymphocytes of the lamina propria have several neuropeptide receptors (such as SP, CGRP, VIP, SOM etc.): during inflammation, immune cells release prostanoids and cytokines, and enteral neurons show receptors for these immune mediators.10 Using immune-histochemical and immunofluorescent methods, it was demonstrated that Toll-like receptors: TLR-3 and TLR-7 recognizing viral RNA, as well as TLR-4 recognizing lipopolysaccharides of Gram (-) bacteria, are represented not only in the submucosal and intermuscular plexus of the GIT, but also in sensor neurons of the posterior horn of the spinal cord [10]. Thus, enteral neurons can react both to inflammatory stimuli and bacterial and viral components participating in the process of host-microbiota interaction. Considering these data, general mechanism of infantile colic can be represented as a “vicious circle”, where the pathological intestinal microbiota play a role with dietary factors causing eccessive gas in infants.11
To evaluate the role of lactase deficiency (LD) and gut microbiota in determining functional disorders (FD) of gastrointestinal tract in early infancy.
Participants
102 full-term breastfed infants or formula fed, aged 10-60 days, were recruited in the unit of Gastroenterology at the Saint-Petersburg State Pediatric Medical University, or for this prospective randomized, double blind, placebo-controlled study. Infants were selected on the basis of their parent’s willingness to participate in the study. We excluded infants with prematurity (gestational age < 36 weeks), birth weight < 2500 grams, chronic disease, allergy or atopic disease, and recent (less than 1 week) exposure to probiotics and antibiotics.
Interventions
Randomisation, allocation concealment, blinding
We recruited 102 infants aged 1 to 6 months.
According to the Rome criteria IV for infants, FD were diagnosed in 62 of them, and they were included in the treatment group. The control group included 40 healthy infants of comparable age. Study design is reported Figure 1.
Inclusion criteria in the treatment group were:
Exclusion criteria were:
The control group included breastfed healthy infants 1 to 6 months without symptoms of any gastrointestinal disorders. The treatment group was divided in two subgroups according to the presence (n= 23 ) or absence (n=39) of lactase deficiency.
Methods
Identification of fecal microbiota content
Fecal microbiota content was identified using 2 procedures: molecular identification methods (PCR with specific primers) and culture. These results allowed us to determine whether some intestinal species differ between colicky and non-colicky infants, and whether some species are more predominant in one group than in the other.
In the treatment group all subjects were affected by infant regurgitation (IR); 44 (71.0%) also presented infantile colic (IC), 3 infants (4.9%) showed constipation (Figure 2). As previously declared, the treatment group was divided in two subgroups according to lactase deficiency: infants belonging to the 1st subgroup (lactase deficiency -LD) were affected by IR and IC, but no one showed signs of constipation. Subjects belonging to the 2nd subgroup (no LD) were affected by IR, but only 21 (53.9%) suffered from infantile colic (IC), 3 (7.7%) from constipation.
IR was present in both subgroups, while IC affected all infants belonging to the first subgroup but only 53.9% of the second one. On the other hand, constipation was found only in infants without lactase deficiency. We analyzed the age-related distribution of gastrointestinal functional disorders in infants 1-6 months old. We observed that IR was the most common manifestation of FD (100% of studied subjects). IC decreases through the months: it was diagnosed in 19 (95.0%) infants 1-2 months old, in 18 ones (72.0%) 3-4 months and only in 7 subjects (41.2%) 5-6 months old. Constipation, on the contrary, appears later in 3 infants (17.7%) 5-6 months old. The combination of two or more FD has been observed in all infants at the age of 1-2 months, in 76.0% of infants at the age of 3-4 months, in 29.5% of infants at the age of 5-6 months. Considering occurrence time: in the treatment group, symptoms of FD appeared as follow: 58.0% during the first month (29% in the first weeks of life), 9.7% in the second month and 3.3% in the third one. In infants affected by lactase deficiency (LD), symptoms have been observed earlier: 21.7% developed them during the first days of life and 78.3% in the first month. In the group without LD only 46.1% of infants developed functional symptoms in the 1st month (33.3% from 2-3d weeks of life); 15.4% of them showed first manifestations from the 2nd month and in 5.2% from the 3d month. Pregnancy and delivery were not physiological in 85% of cases recruited in the treatment group: 26% suffered fetal hypoxia during delivery; concerning mothers, 23% of them were affected by high blood pressure during pregnancy, 23% faced a risk of miscarriage, 10% were affected by urogenital diseases and 3% by nephropathy (Figure 3). In the subgroup of infants with symptoms of FD that were affected by LD, 22 children (95.7%) were born by vaginal delivery and 1 child (4.3%) by the caesarean section. On the other hand, in the subgroup of FD without LD, 21 children (53.9%) were born by the caesarean section (Figure 4). Subjects belonging to the control group (CG) did not experience complications during pregnancy and delivery, while in the treatment group only 56.5% did not showed complications during delivery: 5 mothers (8.1%) received delivery stimulation and 22 (35.5%) were treated with antibiotics. Infants with LD were applied to the breast immediately after birth. On the contrary, in the subgroup without LD, but with FD, only 17 (43.6%) were applied to the breast immediately, 9 (23.0%) in several hours on the first day and 13 (33.4%) were later on. In the treatment group 31 infants (50%) were partially formula fed: 1 subject (4.3%) in the LD subgroup, 30 infants (76.9%) in the other one. Infants in the control group were exclusively breastfed.
Gut microbiota analysis
The analysis of gut microbiota composition, performed through bacterial culture of fecal samples, showed that the colonies of Staphylococcus aureus, Klebsiella pneumoniae and Klebsiella oxytoca were significantly higher in the treatment group than in the control one (Table 1).
Microorganisms |
FD with LD |
FD without LD |
CG |
Bifidobacteria |
11±1 |
10.6±0.3 |
10.0±0.6 |
Lactobacteria |
7±1 |
6.6±0.3 |
6.6±0.3 |
Citrobacter freundii |
4.5±0.5 |
4.5±0.4 |
4.0±1 |
Klebsiella oxytoca |
5.3±0.8 |
4.5±0.4 |
4.7±0.5 |
Klebsiella pneumonia |
5.2±0.7 |
5.5±0.5 |
4.8±0.4 |
Staphylococcus aureus |
4.2±0.4 |
3.0±0.8 |
2.2±0.8 |
Table 1 Composition of fecal microbiota in children with FD in combination with LD or without LD compared with children from the CG based on data of bacteriological examination of feces (lg CFU/g).
Statistically significant differences are marked in bold (p<0.05)
Using real time PCR, less microbial species were detected in the treatment group than in the control one (with no significant differences between the two subgroups). Bifidobacteria were higher in infants without functional disorders. Clostridium difficile and Enterococcusspp. were significantly higher in subjects affected by FD but without LD (Table 2). Fecal Calprotectin analysis: Average value of calprotectin in the treatment group was 344.58±25.32 µg/g, significantly higher than in the control group (82.92±6.47 µg/g) (p<0.001). Infants affected by LD showed higher values of calprotectin (mean 522.21±27.84 µg/g) compared to the other subgroup (239.82±24.35 µg/g) (p<0.001). Fecal calprotectin at the age of 1-2 months and 3-4 months was significantly higher than in infants 5-6 months old. Fecal calprotectin in colicky infants was higher (396.36±27.7 µg/g) than in non-colicky ones (218.00±42.98 µg/g) (p=0.001) (Figure 5).
Microorganisms |
FD with LD |
FD without LD |
CG |
Total count of microbes |
10.1±0.3 |
10.1±0.2 |
10.2±0.2 |
Bifidobacteria |
8.5±0.5 |
8.8±0.3 |
9.4±0.3 |
Lactobacteria |
5.9±0.8 |
6.3±0.8 |
6.2±0.4 |
Bacteroides fragilis |
8.6±0.7 |
8.4±0.5 |
8.4±0.3 |
Clostridium difficile |
4.6±0.8 |
4.9±0.7 |
3.3±0.3 |
Enterococcus spp. |
5.2±0.2 |
5.8±0.2 |
4.7±0.4 |
Table 2 Composition of fecal microbiota in children with FD in combination with LD or without LD compared with children from the CG based on real-time PCR of feces (lg CFU/g).
Statistically significant differences are marked in bold (p<0.05).
Treatment
We employed different approach in the treatment group: breastfed infants with LD received β-galactosidase 700 units at each mealtime for 28 days (subgroup А, n=23), breastfed infants without LD received Lactobacillus reuteri in drops, 5 drops daily (subgroup В, n=19) and formula fed ones without LD were treated with a formula containing Lactobacillus reuteri (subgroup С, n=20) (Figure 1).
In the treatment group, after 28 days, we observed that infantile colic frequency decreased in 49 infants (79.0%), IR frequency reduced in all infants (severity showed a reduction in 17 subjects, 27.4%) and stool frequency reached the frequency of at least once a day in 36 (58.0%). Crying time decreased significantly (table 3), as well as fecal calprotectin values (Figure 6). Infants receiving β-galactosidase showed a significant reduction in IC frequency (p<0.001) and a decreasing of frequency (p<0.001) and grade (p<0.05) of IR during therapy. Before treatment, severe regurgitation was observed in 4 infants (18.2%), and after the treatment no one suffered it. Stool frequency decreased in 16 children (72.7%) and stool samples showed a more physiological pattern of consistence, color, volume and composition than before. Bloating became rare and moderate. The basal level of hydrogen and its increment in 60 minutes after load significantly decreased (p<0.05). In addition, significant decrease of fecal calprotectin values during therapy (p<0.001) was detected.
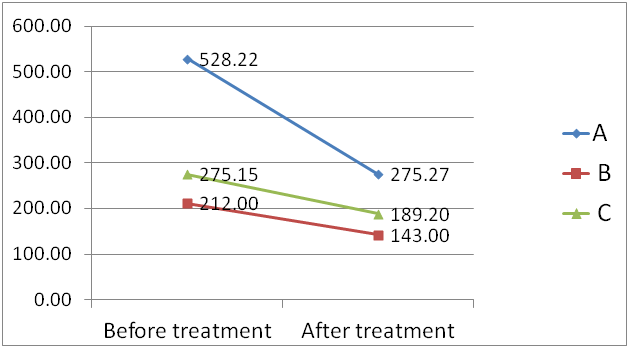
Figure 6Dynamics of fecal calprotectin (mg/g) after treatment with : А- β-galactosidase; В – L.reuteri; C – formula with L.reuteri.
Duration of crying |
А – β-galactosidase (n=23) |
В – L.reuteri in drops (n=19) |
С – formula with L.reuteri (n=20) |
Before treatment |
3.86±0.07 |
2.78±0.40 |
2.61±0.31 |
After treatment |
2.00±0.02 |
1.93±0.30 |
1.34±0.13 |
Significance of |
td=24.89 |
td=7.71 |
td=4.92 |
differences |
p<0.001 |
p<0.001 |
p<0.001 |
Table 3 Dynamics of crying duration in the treatment period.
Microbiota
After the treatment a lower concentration of pathobiontes (CPF), mostly Klebsiella pneumoniaе, Staphylococcus aureus and Clostridium difficile, was observed(p<0.05), (table 4), while, according to PCR of fecal samples, physiological bacteria were increased (mainly Bifidobacteria, Lactobacillus and Bacteroides fragilis).
Microorganisms |
Subgroup А (n=23) |
td |
p |
|||
before treatment |
after treatment |
|||||
Bifidobacteria |
11.00±0.06 |
11.00±0.04 |
0 |
=1 |
||
Lactobacteria |
7.00±0.07 |
6.90±0.09 |
0.88 |
=0.38 |
||
Citrobacter freundii |
4.54±0.27 |
4.30±0.21 |
0.33 |
=0.74 |
||
Klebsiella oxytoca |
5.31±0.42 |
4.72±0.30 |
1.6 |
=0.12 |
||
Klebsiella pneumoniae |
5.13±0.33 |
4.72±0.22 |
2.41 |
=0.02 |
||
Staphylococcus aureus |
4.36±0.12 |
3.80±0.24 |
2.86 |
=0.01 |
||
Table 4 Dynamics of composition of the intestinal microbiota during the treatment with β-galactosidase
Symptoms
During the treatment with L.reuteri in drops, the following changes have been registered: decreasing of IC frequency, regurgitation frequency, stool frequency (with a better consistence and composition of stool samples) and a lower rate of bloating. Baseline level of hydrogen at 30 minute and 60 minute levels significantly decreased (p<0.05).
Calprotectin
Calprotectin value decreased in 4 infants (20%). The employment of L.reuteri drops in breastfed infants without LD led to a significant decrease of Klebsiella pneumoniaе(p<0.05) (table 5), also demonstrated by PCR on fecal samples. An increasing trend of Lactobacillus associated to a lower concentration of Enterococcus spp. and Clostridium difficilewas observed.
Microorganisms |
Subgroup B (n=19) |
td |
p |
|||
before treatment |
after treatment |
|||||
Bifidobacteria |
10.80±0.20 |
9.95±0.49 |
1.63 |
=0.11 |
||
Lactobacteria |
6.85±0.15 |
6.95±0.05 |
0.62 |
=0.54 |
||
Citrobacter freundii |
4.00±0.02 |
3.99±0.01 |
0.45 |
=0.66 |
||
Klebsiella oxytoca |
4.50±0.11 |
4.00±0.28 |
1.66 |
=0.1 |
||
Klebsiella pneumoniae |
6.15±0.34 |
5.05±0.27 |
3.1 |
=0.007 |
||
Staphylococcus aureus |
3.25±0.61 |
4.33±0.27 |
1.21 |
=0.24 |
||
Table 5 Dynamics of composition of the intestinal microbiota during the therapy with L.reuteri
In the last subgroup (treated with a formula containing L.reuteri) a lower incidence of IC, an higher defecation frequency in constipated infants, normal stool consistence and lower rate of bloating were observed. Average Calprotectin value was significantly reduced (p<0.05). The treatment led to a significant decrease of Citrobacter freundii and S.aureus(p<0.05) (table 6) and PCR method revealed a significant reduction of pathobiontes (p<0.05).
Microorganisms |
Subgroup В (n=20) |
td |
p |
|||
before treatment |
after treatment |
|||||
Bifidobacteria |
10.45±0.22 |
9.70±0.35 |
1.59 |
=0.12 |
||
Lactobacteria |
6.45±0.19 |
6.35±0.23 |
0.28 |
=0.77 |
||
Citrobacter freundii |
4.95±0.35 |
4.05±0.05 |
2.16 |
=0.04 |
||
Klebsiella oxytoca |
4.95±0.95 |
4.21±0.16 |
1.56 |
=0.13 |
||
Klebsiella pneumoniae |
4.90±0.39 |
4.68±0.35 |
0.55 |
=0.58 |
||
Staphylococcus aureus |
2.65±0.52 |
2.18±0.48 |
2.67 |
=0.03 |
||
Table 6 Dynamics of composition of the intestinal microbiota at administration of the formula with L.reuteri
Our study has showed that infantile regurgitation (IR) and infantile colic (IC) dominate in the structure of FD of gastrointestinal tract in children of the early age, which corresponds to the results of European studies.1 We revealed statistically significant frequency of perinatal disorders in treatment group compare to control group: mother’s pathology during pregnancy (90.3%), delivery by the caesarean section (35.5%), administration of antibiotics in the process of delivery (35.5%), premature birth (8.1%), additional feeding with infant formula at the maternity hospital (50%) which can be regarded as the risk factors for FD development. These factors may influence the process of formation of the intestinal microbiota as well as permeability of the intestinal barrier, and would be trigger of the immunological response promoting more expressed inflammation of the intestinal mucosa. Alteration of microbial community in infants, as transcriptome analysis has shown, may lead to immunity-related gene expression in epithelial cells.12 According our data, in all children of the early age with FD, the intestinal microbiota is characterized with the increased number of pathobionts. It can cause more expressed inflammation, which is confirmed by the increased level of calprotectin in feaces.
Transitory LD in the first months can cause symptoms, similar to FD in a part of infants. Since, in the clinical aspect, as FD with LD as without, demonstrate a high degree of similarity, establishment of the reason for symptoms is possible only after performance of the hydrogen breath test. It has practical significance, as FD treatment should do considering present LD. After LD has been revealed, administration of β-galactosidase may be recommended, because it can effectively eliminate clinical symptoms of FD, decrease the level of hydrogen in exhaled air and improve the gut microbiota. Our data confirming the efficacy of the probiotic strain L.reuteri in FD, both in the infant formula or in drops, in elimination of FD symptoms, that correlated to gut microbiota changing and fecal calprotectin level as the sign of inflammation in the gut.
How can be high efficacy of L.reuteri in the treatment of colic explained? As in case with the majority of other probiotics, L.reuteri has immunomodulating activity, influences permeability of the intestinal epithelium and renders an anti-inflammatory effect. The latter was confirmed in the experiment with cell cultures of the human intestinal epithelium conducted by D Ma et al.13 The study showed that L.reuteri suppresses stimulated by TNF-α IL-8 secretion both impeding degradation of the inhibiting factor IκB and preventing translocation of the nuclear inflammation factor NFκB to the nucleus, as well as by direct suppression of IL-8 RNA expression. It is important to note that only live bacteria demonstrated this effect; neither killed nor subjected to gamma radiation microorganisms and bacterial lysates demonstrated this activity. The effect depended from the dose of L.reuteri.
The discovered mechanisms of anti-inflammatory effect of L.reuteri are the result of decrease of synthesis of IL-8, the main epithelial stimulator of migration of neutrophils, which leads to the decrease of inflammatory infiltration in the intestinal mucosa.
Anti-inflammatory activity of L.reuteri per se could be sufficient for IC mitigation, but the number of studies showed its direct impact at enteral neurons and motility. In the experiment on mice performed by T Kamiya et al.,14 inhibition of vegetative abnormalities of the heart rate and decrease of activity of ganglions of the posterior horn of the spinal cord caused by extension of the large intestine after administration of L.reuteri were observed. The effect was observed after prescription of both live and killed bacteria. X Ma et al.15 demonstrated that preliminary 9-day administration of L.reuteri in healthy mice decreased the potential of activity of ganglions of the posterior horn caused by extension of the large intestine. B Wang et al.16 showed that the use of L.reuteri in experimental animals decreased intestinal motility and hyperpolarization of muscle cells because of inhibition of Са-activated potassium channels. Dose-dependent decrease of activity of motor complex of the intestine in the reaction to live bacteria has been showed. Killed L.reuteri and other Lactobacillus (L.salivarius) did not cause any effect. Results observed in these studies may be due to the direct impact of L.reuteri on enteral ganglions, afferent and efferent signaling pathways.
Probiotic bacteria in our gut communicate with the CNS and regulate brain neurochemistry and behavior in a number of different ways. These mechanisms include production of bacterial metabolites and immune mediators, such as cytokines, and signaling to the brain directly via the vagus nerve.17
Thus, the treatment effect of L.reuteri in FD is the result of the combination of the anti-inflammatory activity and stimulation of some sensitivity and pain perception, along with regulation of motility reactions. This combination of several mechanisms acts on the key factors of FD pathogenesis: dysbiosis, inflammation, hyperalgesia, motility disturbance. Cure of these main mechanisms can indirectly correct other important issues: increased permeability of the intestinal barrier as well as disturbance of food digestion and production of unfavorable metabolites and gases.
For detection of significance of lactase deficiency (LD) and microbial disturbances in functional disorders (FD) in infancy, we examined 102 infants from 1 to 6 months. According to Rome criteria III for infants, FD have been diagnosed in 62 infants. Hydrogen breath test (HBT) with lactose, fecal culture and real time PCR, fecal calprotectin have been assessed in all infants before and after 28 day treatment. In 37% of patients with FD, lactose intolerance (LD) has been detected. In 85% of mothers of patients with FD pregnancy pathology was registered, more common were: fetal hypoxia– 26%, gestosis – 23%, risk of miscarriage – 23%, urogenital pathology – 10%, nephropathy – 3%. There was additional formula feeding in the first days of life at the maternity hospital just in 50% infants with FD. In all patients with FD, disbiosis with increased amount of pathobionts has been revealed. The level of fecal calprotectin was increased in all patients with FD. In infants with LD, the positive dynamics has been achieved after administration of β-galactosidase; in FD children without LD after administration of L.reuteri both in infant formula and in drops. These probiotic effectively eliminated FD symptoms, decreased the level of calprotectin and improved microbiota.
None.
Author declares no conflicts of interest.

©2019 Alexandrovna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.