eISSN: 2373-6372


Research Article Volume 3 Issue 3
1Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences of Niigata University, Japan
2Department of Internal Medicine, The Nippon Dental University, Japan
Correspondence: Shogo Ohkoshi, Department of Internal Medicine, School of Life Dentistry at Niigata, The Nippon Dental University, 1-8 Hamaura-cho, Chuo-ku, Niigata 951-8580, Japan, Tel +81 25 2118243, Fax +81 25 267 1582
Received: November 17, 2015 | Published: December 21, 2015
Citation: Yano M, Ohkoshi S, Hara H, Hirono H, Watanabe K, et al. (2015)Interferon- β Augments the Effects of Cisplatin on Hcc Cell Lines by RegulatingSub Cellular Localization of P21. Gastroenterol Hepatol Open Access 3(3): 00079. DOI: 10.15406/ghoa.2015.03.00079
Introduction: Interferon (IFN) has been used for patients with advanced hepatocellular carcinoma (HCC) in combination with chemotherapeutic agents. We previously reported that IFN-βinduced a shift into the nucleus of Cytoplasmic p21 (Cip1/WAF1), known as a tumor-suppressor gene, and contributed to the prevention of HCC.
Aims: To use hepatoma cell lines to compare the growth-suppression activity of IFN-α and β, in the context of enhancing cisplatin’s effects.
Results: IFN-β caused a significant nuclear localization of p21 when compared to IFN-α IFN-β augmented the cytotoxic effects of cisplatin more than IFN- α. Notably, when IFN-α was used only for pre-treatment, significant apoptosis with subsequent cisplatin treatment was observed.
Conclusion: Results suggest that IFN- β enhances the cytotoxic effects of cisplatin more than IFN-α, partly because of its effect of keeping p21 in the nucleus. Thus, IFN-β-based chemo-therapeutic regimen might provide more effective outcomes of HCC treatment.
Keywords: hepatocellular carcinoma, p21 (Cip1/WAF1), IFN- β, Cisplatin, subcellular localization, Apoptosis
Combination of IFN-α with chemotherapeutic agents is a common therapy for advanced HCC, although the effects are not significant in most patients. We compared the anti-proliferative effects of IFN-β and -α and found that IFN-β enhanced the cytotoxic effects of cisplatin in HCC cell lines than IFN-α. This phenomenon was partly explained by its effect of keeping tumor suppressor protein p21 in the nucleus, inducing apoptosis. A new therapeutic strategy for IFN-included chemotherapy for HCC is suggested.
Although induction of p21 (also known as Cip1/WAF1) as a universal inhibitor of cyclin/CDK activity downstream of active p53 generally results in inhibition of cell proliferation, recent studies have suggested the opposite effect of p21; that is, one that renders normal cells susceptible to transformation by conferring an anti-apoptotic or a cell-proliferative status.1,2. It has been suggested that the intracellular distribution pattern of p21 in particular may determine whether it has anti-oncogenic oroncogenic property. Namely, nuclear p21 induces apoptosis, while Cytoplasmic p21 is anti-apoptotic and oncogenic.1–3
IFN-α and -β are type-I IFNs;4 both bind a common cell-surface receptor (IFNAR), which comprises high-affinity (IFNAR2) and low-affinity (IFNAR1) components. Although the IFN-α subtypes and IFN-β use the same IFNARs, they exhibit functional differences. IFN-β is more potent than IFN-α for inducing the apoptotic and anti-proliferative pathways required for control of tumor cell growth.5
Combination therapy of IFN-α and chemotherapeutic drug, such as 5-fluolouracil, has been used for patients with advanced HCC.6 However, clinical effects were not satisfactory and did not improve the prognosis of most patients with this severe illness.
We previously reported that IFN-β induced a shift into the nucleus of Cytoplasmic p21 and contributed to the prevention of HCC.7 Here we report that the in vitro combination of IFN-β and cisplatin had a higher apoptosis-inducing effect than IFN-α and cisplatin, and that the greater effect of IFN-β to shift p21 from the cytoplasm into the nucleus may partly explain this phenomenon.
HepG2 and Hep3B were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) and antibiotic-antimycotic solution (Sigma Aldrich, St. Louis, MO). IFN-α and -b were kindly provided by Schering-Ploughand Toray Medical Co., Ltd. respectively (Japan). The number of viable cells was determined by trypan blue staining Triplicate cell cultures were maintained and used for assays.
Proteins were isolated from cell lines and the 10µg aliquots were separated by SDS-PAGE and subjected to Western blot analysis. Nuclear and cytoplasmic fractions of culture cells were prepared by NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s protocols.
Rabbit-polyclonal antibodies specific for p21, anti-cleaved caspase 3, 9, and p53 were purchased from Cell Signaling Technology (Beverly, MA). Mouse-monoclonal antibodies specific for b-actin were purchased from Sigma, and other specific antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Effects of cisplatin on hepatoma-derived cell growth
Cytotoxic effects of 2µg /mL cisplatin added to the culture medium were confirmed in both hepatoma-derived HepG2 (wild p53) and Hep3B (null p53) cell lines (Figure 1). Time-dependent decreases in viable cells were observed in both cell lines.
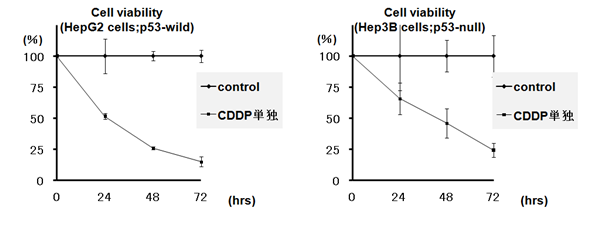
Figure 1 Time course analysis of cytotoxic effects of cisplatin on hepatoma-derived cell lines. The number of viable cells was determined by try pan blue staining.
Comparison of IFN-α and-β for their effects on intra-cellular localization of p21
We previously showed that IFN-β induced nuclear-dominant localization of p21 in hepatoma cell lines.7 We compared the effects of the same doses (100 IU/ml) of IFN-α and –β on p21 intracellular localization (Figure 2). Without treatment, most p21 protein was located in the cytoplasm. However, treatment with IFN-β induced a significant shift of p21 to the nuclear fraction when compared to the effect of the same dose of IFN-α. Intracellular localizations of Hsp90 (supposed to be cytoplasmic) and p53 (supposed to be nuclear), were not changed. In addition, phosphorylated (activated) Stat 1 was induced more in IFN-β- treated cells than in those treated with IFN-α.
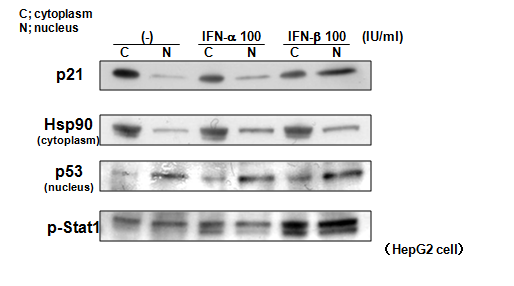
Figure 2 Comparison of the effects of IFN-α and -β on the intracellular localization of p21. IFN-β caused a more significant shift of p21 to the nucleus than did IFN-α. Note that intracellular localizations of Hsp90 and p53 were not changed.
Comparison of effects of different IFN-including treatment protocols on the cytotoxic effects of cisplatin
The effects of different IFN-α or –β treatments on cisplatin cytotoxicity were subsequently tested (Figure 3). Experimental conditions were as follows: 1, control: 2, only cisplatin (2µg /ml); 3 and 5, IFN-α or-β (100 IU/ml),respectively, for pretreatment only, before cisplatin-only treatment; 4 and 6, IFN-α or-β concurrent with cisplatin treatment (Figure 3A). IFN-α did not enhance the cytotoxic effects of cisplatin, whereas IFN-β did. Notably, enhancements of cisplatin’s effects by IFN-β were observed even when IFN-β was added only before cisplatin-only treatment, indicating it sensitized cells to cisplatin-induced apoptosis (Figure 3B). The effects of this sensitization protocol were comparable to those of incubation with IFN-β and cisplatin together for the full time period, especially in HepG2 cells. Significant induction of cleaved caspase 3 and 9, which demonstrates apoptosis, was observed when cells were treated with IFN-β either as pretreatment only (lane 5) or in conjunction with cisplatin treatment (lane 6) (Figure 3C); this strong induction of apoptotic proteins was consistent with the results of the cell-viability tests.
It has been reported that Cytoplasmic p21 interacts with and inhibits apoptotic procaspase-38 or apoptosis-signal-regulating kinase 1.9, while nuclear p21 localization is associated with pro-apoptotic function.2. Thus, the shifting of subcellular localization of p21 may be the clearest way to explain its functional versatility. Our past study showed that hepatitis B virus X (HBx) gene caused Cytoplasmic localization of p21 and that IFN-β returned this Cytoplasmic p21 to the nucleus, contributing to the prevention of HBx-induced HCC.7. Because IFN-β is considered to be more potent than IFN-α in anti-proliferative functions,10 we first attempted to compare their effects on the intracellular shifting of p21, and found that IFN-β effected a greater shift of p21 to the nucleus compared with IFN-α. This phenomenon may partly explain the strong anti-proliferative effects of IFN-β.
We then attempted to determine which IFN is more effective in inducing apoptosis when combined with cisplatin, the drug that has been used most widely for HCC patients mainly via hepatic arterial infusion.11 As expected, IFN-β was more potent apoptosis inducer than IFN-α consistent with results of the p21 localization experiments. We then speculated that, because IFN-β caused significant nuclear accumulation of p21, its use as a pretreatment only may be sufficient to enhance the apoptosis-inducing effect of subsequent cisplatin treatment; the results of the appropriate experiments described above confirmed this theory. Thus pre-incubation with IFN-β sensitized HCC cells, possibly by inducing strong nuclear accumulation of p21, and subsequent cisplatin treatment caused significant levels of apoptosis. Koster et al.12 showed that localization of p21 in the cytoplasm was critical for cisplatin resistance in testicular cancer.12 The current experimental observations suggest promising efficacy of IFN-β, and also suggest the possibility that the total cisplatin dose could be reduced while remaining effective for clinical use.
We confirmed that in HCC cell lines, IFN-β even when used for pretreatment only, was a more potent enhancer of cisplatin’s effects than was IFN-α, at least partly because of its stronger effect of shifting p21 into the nucleus. By this mechanism, IFN-β sensitized cells to the chemotherapeutic effects of cisplatin. The current findings shed light on the clinical importance of IFN-β in the context of anti-cancer therapy and advance understanding of the chemotherapeutic mechanisms in the combined use of IFNs and anti-cancer agents.
None.
Author declares there are no conflicts of interest.
None.

©2015 Yano, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.