eISSN: 2373-6372


Research Article Volume 11 Issue 1
1Department of Gastroenterology, Saint Petersburg State Pediatric Medical University, Russia
2Department of Internal Medicine, First Pavlov State Medical University of St. Petersburg, Russia
3Department of Algorithmic Biotechnology, Saint Petersburg State University, Russia
4Group of Companies “Craft”, Russia
Correspondence: Elena A Kornienko, Doctor of Medicine, Professor, Head of Gastroenterology Department of Saint Petersburg State Pediatric Medical University; 2 Litovskaya Str. St.Petersburg 194100, Russia
Received: November 29, 2019 | Published: January 16, 2020
Citation: Kornienko EA, Parolova NI, Ivanov SV, et al. Gastric microbiota and probiotics opportunities in helicobacter pylori eradication in children. Gastroenterol Hepatol Open Access. 2020;11(1):13-23. DOI: 10.15406/ghoa.2020.11.00407
H. pylori infection changes the composition of gastric microbiota, but data in children are scared. Probiotics can improve the results of eradication therapy, but as long as adjuvant therapy.
The aim of our study to assess effectiveness of monotherapy and adjuvant therapy of probiotic L. reuteri DSMZ17648 (Lr) in H. pylori (+) children.
Material and methods: In 103 patients 9 to 17 years, a double-blind, randomized, placebo-controlled study of the effectiveness of L. reuteri DSMZ17648 (Lr) was performed both as monotherapy and adjuvant to eradication therapy (omeprazole + amoxicillin + metronidazole + bismuth- OAMB for 10days), the Lr course in subgroup A lasted for 28days, in subgroup B for 56days. The control НР-status was performed twice: before the treatment and after it (on 56 day) - by endoscopy, histology, Rapid Urease Test (RUT), Ammonia Breath Test (ABT), H. pylori count in the biopsy, inflammatory and atrophic indices were counted. In 8 H. pylori (+) and 8 H. pylori (-) children, sequencing of 16S-rRNA microbiota in biopsies of gastric mucosa was carried out for surrounding microbiota assessment.
Results: H. pylori decreased microbial diversity associated with inflammation and focal mucosal atrophy. H. pylori eradication was achieved on Lr monotherapy in 50% after 28 and 60% after 56days, on adjuvant Lr therapy - 60% after 28 and 77.8% after 56days, in placebo group – 68.8% after 28 and 56days. Clinical manifestation, inflammatory and atrophic changes during Lr treatment significantly and reliably decreased.
Conclusion: Lr monotherapy is comparable in effectiveness to a standard eradication therapy.
Keywords: pylori, non-Helicobacter gastric microbiota, probiotics, L. reuteri
The stomach was long considered as almost sterile organ due to hydrochloric acid production. However, the discovery of H. pylori showed the existence of microorganisms adapted not only to survival in an acidic environment, but also to colonization of this particular part of digestive tract.1
The emergence of modern molecular genetic methods based on bacterial 16S-rRNA genes amplification allowed to obtain more comprehensive information on the representatives of the gastric microbial community.2 It turned out that gastric microbiota is much more diverse than it had been previously thought: several modern studies identify more than 200 bacterial phylotypes.3
It is possible that changes in the gastric microbial environment around H. pylori may affect the activity of chronic gastritis initiated by itself, as well as the progression of atrophy, and the risk of developing gastric cancer. It is generally possible to achieve changes in the gastric microbiota with probiotics.
Probiotics can have a direct antagonistic effect on H. pylori due to their metabolites. The amount of lactic acid secreted by Lactobacillus, Bifidobacterium and Pediococcus (50-156mmol) correlates with the intensity of the inhibitory effect on H. pylori.4 Therefore, microbes that produce lactic acid in large quantities – for example, L. salivarius and L. casei Shirota, suppress H. pylori and reduce the activity of inflammation in the stomach, which was demonstrated in an experiment on mice.5
Anti-Helicobacter action is also exerted by bacteriocins - small thermostable proteins synthesized by certain bacteria, in particular, lactobacilli. Kim et al.6 evaluated the anti-Helicobacter activity of seven bacteriocins produced by lactic acid bacteria (nisin A, pedicin PO2, leukocin K, and various types of lacticins). The lactacins A164 and BH5, produced by Lactococcus lactis, showed the greatest inhibitory activity against some strains of HP, that is, the strain specificity of bacteriocins was demonstrated. Lysates of L. acidophilus CRL639, due to the bacteriocins contained in them, completely killed H. pylori after 48 hours exposure.7
Culture supernatant of the L .johnsonii La1 probiotic strain inhibits both urease activity and H. pylori growth.8 This is conditioned by the production of L .johnsonii bacteriocin - lactacin F, and is manifested even when H. pylori is adhered to the culture of epithelial cells, it does not depend on H. pylori toxicity and pathogenicity island presence. Prescription of supernatant L. johnsonii La1 to adult H. pylori (+) patients, significantly reduced the results of C13-breath test.
Similar results were obtained by Coconnier et al.,9 as they incubated H. pylori with L. acidophilus LB supernatant: H. pylori survival rate, its urease activity and adhesion to the epithelium were reduced correspondingly to L. acidophilus LB dosage. This effect did not depend on lactic acid and pH, did not decrease after boiling. H. pylori bacteria looked morphologically defective after incubation with L.acidophilus LB supernatant, changing the spiral shape to U-shaped and decreasing the size, which corresponds to pre-coccoid modification of H. pylori. However, the anti-Helicobacter action of probiotic bacteria remains strain-specific. Thus, the supernatant L. rhamnosus GG had no effect on H. pylori.10
Probiotics reduce inflammation activity in the stomach. This was observed for L. acidophilus LB and L. johnsonii La, L. salivarius WB1004, L. rhamnosus R0011, L. acidophilus R0052 and L.gasseri 0ll2716.11 The intake of yogurt containing L.gasseri protected experimental animals from acute chemical gastritis development, in contrast to milk.11 The intake of yogurt was accompanied by an increase in prostaglandin E2 in the gastric mucosa. Protective action by increasing prostaglandins and growth factors (EGF and bEGF) has also been demonstrated in probiotic strains of bifidobacteria: B. breve and B.bifidum, their administration inhibited ulceration of the gastric mucosa in rats.12 Even the administration of bifidobacterial polysaccharides had a similar anti-ulcer action, this effect depended on the amount of polysaccharides containing rhamnose.13
Attempts to improve the effectiveness of eradication with probiotics have been undertaken in a number of studies. Most of them directed at the evaluation of probiotic therapy with standard eradication regimens. Meta-analysis of 10 randomized trials involving 963 patients who received functional nutrition with probiotics additionally to standard anti-H. pylori therapy and 465 patients on standard therapy showed that probiotics increase H. pylori eradication by 5-15% and reduce the severity of side effects of standard therapy.14
Several studies assessed the efficacy of probiotic monotherapy of H. pylori. M Gotteland et al.15 compared the efficacy of monotherapy S. boulardii, monotherapy L. acidophilus LB and standard 7-day triple regimen in H. pylori (+) schoolchildren. Probiotics were given twice a day for 2 months. Eradication of HP was achieved in 66% of children receiving a triple regimen, 12% for patients receiving S. boulardii monotherapy and 6.5% for L. acidophilus LB monotherapy. The intake of a fermented milk product containing L. johnsonii La1 (LJ1) by 50 asymptomatic volunteers with chronic gastritis for 16 weeks was accompanied by a significant decrease in inflammation activity in the gastric mucosa and colonization density of H. pylori. On the background of LC1 medication, thickening of the mucous layer was also recorded.16
Promising results were demonstrated in a study on the effectiveness of spray-dried L. reuteri DSMZ17648, in which 22 H. pylori infected people participated. H. pylori density level was determined using a standard13 C-urease breath test prior to treatment and after 14days. A significant decrease in the13 C level was achieved in the main group, whereas in the control placebo group the result was not different from the baseline.17 L. reuteri DSMZ17648, unlike other strains, specifically coaggregates H. pylori in the in vitro experiments and keeps this ability even after lyophilisation.
We have conducted a double-blind, randomized, placebo-controlled trial of the efficacy of spray-dried L. reuteri DSMZ17648 ((Pylopass™/Lonza, Germany) in H. pylori-associated gastritis in children, both as monotherapy for 28 and 56days, and as adjuvant with standard eradication therapy, and evaluated gastric microbiota composition according to 16S RNA sequencing in some patients. This specific strain was found by screening hundreds of Lactobacilli strains of a large culture collection (Organobalance GmbH, Berlin, Germany).18
Materials and design of the study
The study was conducted at the gastroenterology department of St. Petersburg Children's City Clinical Hospital No.5 (St. Petersburg, Russia), which is the base of the St.Petersburg Pediatric Medical University, in 2015-2017. In the study were included children, admitted for upper endoscopy because of symptoms of dyspepsia (recurrent pain and discomfort in the upper abdomen). The ethical committee of St. Petersburg Pediatric Medical University 03.04.2015 approved the design of the study. Inform consent was signed by parents of every child before inclusion in the study.
Following upper endoscopy and verification of H. pylori status, 103 children (55 boys and 48 girls) aged 9 to 17 years with histologically confirmed H. pylori-associated chronic gastritis were randomized into 3 groups according the treatment regimen used.
At the primary examination of all H. pylori (+) patients in 8 people who had not previously received antibiotics and probiotics, additional biopsy samples of the gastric mucosa were taken to evaluate microbiota composition by sequencing 16S-rRNA. To compare the composition of gastric microbiota in the absence of H. pylori, 8 children were also included in the study, who had no H. pylori after the upper endoscopy and verification of H. pylori status. See the Study Design in Figure 1.
Three groups of H. pylori (+) patients received the following therapy
Group 1 (n=37) - L. reuteri DSMZ17648 monotherapy: 1 capsule (200 mg) twice a day with meals. This group is divided into two subgroups: 1A and 1B; patients of subgroup 1A (n=17) received treatment for 28days, subgroups 1B (n=20) – for 56days.
Group 2 (n=32) - omeprazole + amoxicillin + metronidazole + three potassium bismuth di citrate (OAMB) regimen for 10days. The medication was administered at average age-appropriate dosage according to ESPGHAN recommendations. From the first day of treatment, patients received a placebo in similar capsules that did not contain a probiotic, 1 capsule twice a day during meals. This group was also divided into two subgroups: 2A and 2B: patients of subgroup 2A (n=16) received placebo for 28days, subgroups 2B (n=16) – for 56days.
Group 3 (n=34) – omeprazole + amoxicillin + metronidazole + three potassium bismuth di citrate (OAMB) regimen lasting 10days, in average age doses. From the first day of this standard treatment patients received L. reuteri DSMZ17648 1 capsule (200 mg) twice a day with meals. This group was also divided into two subgroups: 3A and 3B: patients of subgroup 3A (n=16) received the L. reuteri DSMZ17648 for 28days, subgroups 2B (n=16) – for 56days. Neither the patients, nor the investigators knew which capsules – probiotic or placebo – the patients got; this information was recovered just after study finished.
Before the start and after the end of the treatment (on day 0 and day 56), all children underwent upper endoscopy with 4 biopsy specimens taken from antrum and corpus of the stomach followed by a histology and Rapid urease test RUT Helpil®. This test is based on a solid carrier (provided by AMA Ltd, St. Petersburg, Russia), allowing for 3-minute assess of H. pylori presence by urease activity, changing the indicator colour from yellow to blue.18 Our long-term (over 10years).19 experience with RUT Helpil® in children has demonstrated its high sensitivity (87.3%) and specificity (93.6%). Other authors also note the high sensitivity and specificity of RUT Helpil ®.21,22
For histological evaluation we used two stains: hematoxilin-eozin and azur-eozin. We scored two indexes: assessed the degree (mild, moderate, severe) of mononuclear infiltration, neutrophil infiltration, oedema and scored the sum of them as Inflammatory Index (II); atrophy of glands, fibrosis and intestinal metaplasia (mild, moderate, severe) were determined and scored the sum of these signs as Atrophy Index (AI).23 H. pylori density was also assessed and quantified.
Indirect assessment of urease activity in the stomach was performed by Ammonia Breath Test (ABT) Helic® (AMA Ltd., St. Petersburg, Russia) three times: before the therapy and ondays 28 and 56 after the start of the therapy. To perform this test, we got automatic air sampling from oral cavity before and after consumption of unlabelled urea 12C (500mg in 50ml of water) through indicator tube and assessed result by increase in the length of the stained column in the tube (in mm).24,25 Our experience with ABT Helic ® in children shows that with strict adherence to the procedure, its sensitivity is 94-97%, and specificity is 92-96%.26
The use of several valid methods for H. pylori verification allowed a more objective assessment of the effectiveness of the therapy. The design of the study is presented in Figure 2.
To study the gastric microbiota composition in H. pylori-infected children, in comparison with those not infected prior to start of the therapy, we determined the bacterial metagenome of the biopsy specimens of the gastric mucosa. In 16 children: 8 H. pylori (+) and 8 H. pylori (-), during the endoscopy, 1 biopsy specimen from the antrum of the stomach and in 2 children, from the corpus of the stomach, additionally taken to study their bacterial composition by sequencing fragment of the 16S rRNA gene. All biopsy specimens were placed immediately in a plastic test tube with EDTA, frozen and stored at −80 °C until tested. DNA was isolated from the specimens using the QIAamp DNA Mini Kit (Qiagen), as per the manufacturer’s instructions.
The libraries for 16S rRNA genes were constructed by amplifying specific DNA-region using FastStart Taq DNA Polymerase (Roche, Switzerland). The primers were specific for V4 and V5 regions and contained barcoded adapters for sequencing on Ion Torrent PGM (Table 1). We ran amplification in 25mkl of mixture, containing 50mM Tris/HCl, 10 mM KCl, 5 mM (NH4)2SO4, 4 mM MgCl2, PCR-primers in concentration of 0.4 mkM, each, 0,2mM dNTP, 5U FastStart Taq DNA Polymerase and 5 ng of DNA. The reaction conditions were as follows: 2minutes at 95°C, followed by 35 cycles of 30s at 95°C, 30s at 52°C and 30s at 72°C, and the finishing step was 5minutes at 72°C.
|
Forward primer name |
Forward primer sequence |
Reverse primer name |
Reverse primer sequence |
|
V4F1 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGCTAAGGTAACGTGCCAGCMGCCGCGGTAA |
V5R |
CCTCTCTATGGGCAGTCGGTGATCCGTCAATTCMTTTRAGT |
|
V4F2 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTAAGGAGAACGTGCCAGCMGCCGCGGTAA |
||
|
V4F3 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGAAGAGGATTCGTGCCAGCMGCCGCGGTAA |
||
|
V4F4 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTACCAAGATCGTGCCAGCMGCCGCGGTAA |
||
|
V4F5 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGCAGAAGGAACGTGCCAGCMGCCGCGGTAA |
||
|
V4F6 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGCTGCAAGTTCGTGCCAGCMGCCGCGGTAA |
||
|
V4F7 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTTCGTGATTCGTGCCAGCMGCCGCGGTAA |
||
|
V4F8 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTTCCGATAACGTGCCAGCMGCCGCGGTAA |
||
|
V4F9 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTGAGCGGAACGTGCCAGCMGCCGCGGTAA |
||
|
V4F10 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGCTGACCGAACGTGCCAGCMGCCGCGGTAA |
||
|
V4F11 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTCCTCGAATCGTGCCAGCMGCCGCGGTAA |
||
|
V4F12 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTAGGTGGTTCGTGCCAGCMGCCGCGGTAA |
||
|
V4F13 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTAACGGACGTGCCAGCMGCCGCGGTAA |
||
|
V4F14 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTTGGAGTGTCGTGCCAGCMGCCGCGGTAA |
||
|
V4F15 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTAGAGGTCGTGCCAGCMGCCGCGGTAA |
||
|
V4F16 |
CCATCTCATCCCTGCGTGTCTCCGACTCAGTCTGGATGACGTGCCAGCMGCCGCGGTAA |
Table 1 The specific primers for V4 and V5 regions and barcoded adapters for sequencing on Ion Torrent PGM
After amplification the equal amounts of PCR-products were pooled together and then the mixture was separated on 2% agarose gel. The target DNA fragment with the size about 500bp was extracted using GeneJET Gel Extraction Kit (Thermo Scientific, USA). Resulting DNA library was sequenced on IonTorrent PGM sequencer, using Ion PGM™ Template OT2 400 Kit (Thermo Fisher Scientific, USA). Sequencing was conducted at Research Resource Center for Molecular and Cell Technologies, Research Park, Saint-Petersburg State University (St.Petersburg, Russia).
Resulting data were analyzed using QIIME v. 1.9.0 software. Sequence reads were processed to remove low quality, short, or chimera reads. After quality trimming and filtering, a total of 2,67M reads were included into the analysis, ranging from 2514 to 750050 reads per sample. The amount of reads per sample was reduced to the lowest value (2514) for diversity comparison between samples.
Statistical processing of the research results:
Statistical analysis of the study data was carried out using the statistical software package SPSS 17.0 (SPSS Inc., USA).
The correspondence of the actual distribution of quantitative data to the normal (Gaussian) distribution was verified using the Shapiro-Wilk test. Since in all groups the actual distribution of quantitative data was significantly different from the normal one, median and quartiles were used for descriptive statistics, and nonparametric criteria were used for statistical analysis.
To compare independent (unrelated) observations, the Mann-Whitney test was used; the Wilcoxon test was used to compare paired observations.
To estimate the onset rate of the therapy effect (abdominal pain relief), the Kaplan-Mayer method was used, a comparison of the schedules of the "survival" functions was carried out using a logistic criterion, the Breslow criterion, and the Tarone-Ware criterion. To compare the frequency of adverse events in the comparison groups, the Pearson χ2 criterion was used.
Bioinformatic processing of the sequencing results was carried out using the QIIME package, cluster analysis was used to estimate the distribution of microbiota.
Results of gastric metagenome study:
Based on 16S-rRNA sequencing data, H. pylori was detected in 8 of the 16 gastric samples (1_1, 1_2, 7_1, 10_1, 13_1, 14_1, 8_1, 9.1) – H. pylori (+). In the remaining 8 gastric samples H. pylori was not detected (2_1, 2_2, 3_1, 4_1, 5_1, 6_1, 11_1, 12_1) – H. pylori (-).
Analysis results of taxonomic composition of gastric microbiome of H. pylori (+) children
The analysis showed heterogeneity in taxonomic composition of gastric microbiota in the children examined, see Figure 3.
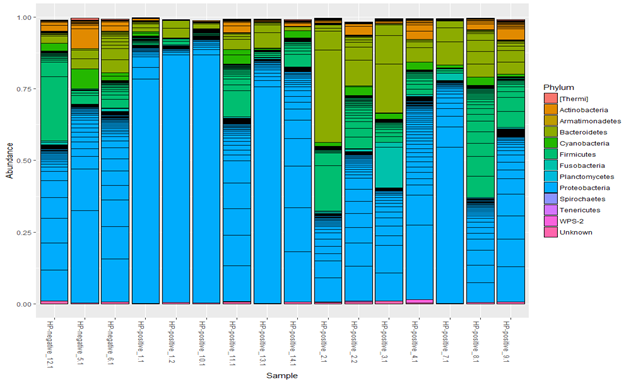
Figure 3 Taxonomic composition of the gastric microbiota in children with chronic gastritis (n=16) at the phylum level.
The dominant stomach bacterial phyla in children with chronic gastritis were Proteobacteria, Bacteroidetes, Firmicutes, whereas Actinobacteria, Cyanobacteria, Fusobacteria were represented to a lesser extent. In all children, regardless of the presence of H. pylori, representatives of Proteobacteria prevailed. However, in H. pylori (+) patients H. pylori averaged 64.1% of the total microbiome, and among Proteobacteria reached 75-99%.
The number of clones of other bacteria in this case was significantly lower, and species diversity was also reduced. This is more noticeable in the bar graphs of the representation of taxa at the class level. Figure 4 in 5 of the 8 H. pylori (+) patients (samples 1_1, 1_2, 7_1, 10_1, and 13_1), H. pylori significantly dominated both among all microbes and among Proteobacteria. As shown in Fig. 4, samples 1_1, 1_2, 10_1, 13_1 and 7_1 are distinguished by a high proportion of the Epsilonproteobacteria class. At the level of orders, these samples retain a shift towards Campylobacterales, at the level of families – towards Helicobacteraceae, at the level of genera – towards Helicobacter.
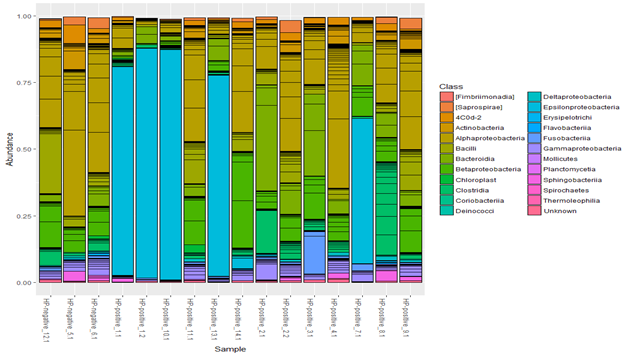
Figure 4 Taxonomic composition of the gastric microbiota in children with chronic gastritis (n=16) at the class level.
The microbial composition of the gastric mucosa in one of the patients H. pylori (+) patients (sample10_1) is shown in Figure 5.
It should be noted that endoscopy in all 5 patients with H. pylori predominance showed pronounced nodularity in the antrum part of the stomach, and according to the histological examination - a high degree of H. pylori density (more than 50 microbial bodies in the field of vision). These patients showed a high activity of inflammation, expressed lymphoid hyperplasia, and signs of focal atrophy in the antral stomach in 3 of the 5 patients.
In 3 H. pylori (+) patients 14_1. 8_1,9_1, according to sequencing, H. pylori was not the dominant microbe, representation of other microbes was more diverse, including the ones within Proteobacteria. Endoscopically and histologically, the signs of inflammation in the stomach were mild; histologically the low degree of H. pylori density (less than 20 microbial bodies in the field of vision) with a predominance of coccoid forms was noted. Microbial composition of the gastric mucosa of one of these patients (sample 14_1) is shown in Figure 6.
Results of taxonomic analysis of the gastric microbiome of H. pylori (-) children:
Endoscopically in H. pylori (-) patients, minimal superficial gastritis predominated, histologically chronic inflammation had low activity. Microbiological composition of the gastric biopsies with the prevalence of Actinobacteria, Alphaproteobacteria and Bacteroidetes phyl. in H. pylori (-) patients (5_1, 6_1, 12_1) was not significantly different from H. pylori (+) biopsy specimens with minimal amount of H. pylori (14_1. 8_1,9_1), but significantly differed from patients with a high H. pylori abundance (samples 1_1, 1_2, 7_1, 10_1, 13_1). In cases with high H. pylori abundance, the dominated gastric phylum was Proteobacteria.
Composition of gastric microbiota at the order level (Figure 7) in H. pylori (-) patients showed more bacterial diversity then in H.pylori (+). In H.pylori (-) patients dominated orders were Actinomycetales, Bacteroidales, Bifidobacteriales and Sphingobacteriales, but in H. pylori (+) patients absolutely dominated Campylobacterale.
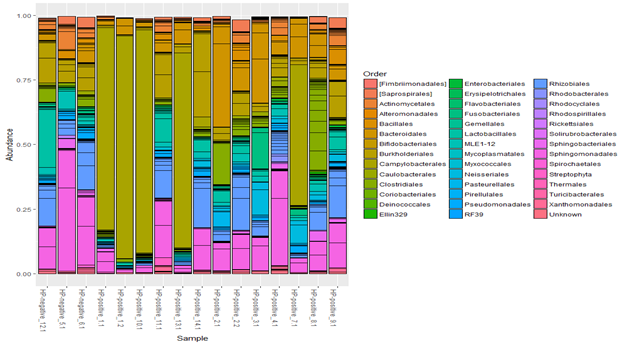
Figure 7 Taxonomic composition of gastric microbiota of children with chronic gastritis (n=16) at the order level. Cluster analysis (Figure 8) demonstrates that composition of the H. pylori (+) microbiota forms two clusters: a separate one (patients with H. pylori predominance), and another one close to H. pylori (-) patients – patients with a low degree of H. pylori. Children with a low amount of H. pylori had a shorter history of the disease, so the surrounding microbiome has not yet undergone significant changes, and the morphological signs of chronic gastritis were still minimal.
Evaluation of L. reuteri DSMZ 17648 effectiveness as an adjuvant and monotherapy for H. pylori infection
According to the results of our double-blind, randomized, placebo-controlled study, the highest percentage of H. pylori eradication was achieved in patients who received a 10-day eradication regimen (OAMV) in combination with L. reuteri DSMZ17648 probiotic for 56days (Table 1). H. pylori eradication was achieved in children of this subgroup in 77.8%. The effectiveness of combination of the traditional regimen of eradication therapy with placebo, irrespective of the duration of treatment (28 or 56days), was 68.8%.
L. reuteri DSMZ17648 monotherapy lasting 28days was effective in 50%; prolongation of this monotherapy to 56days increased the percentage of H. pylori eradication up to 60% (Table 2).
|
Group |
Therapy Regimen |
Duration of treatment (days) |
Number of patients (n) |
Eradication Effectiveness (%) |
|
1A |
L. reuteri DSMZ17648 |
28 |
17 |
50 |
|
1B |
L. reuteri DSMZ17648 |
56 |
20 |
60 |
|
|
||||
|
2A |
Eradication therapy (О+А+М+В) + placebo |
10
28 |
16 |
68,8 |
|
2B |
Eradication therapy (О+А+М+В) + placebo |
10
56 |
16 |
68.8 |
|
|
||||
|
3A |
Eradication therapy (О+А+М+В) + L. reuteri DSMZ17648 |
10
28 |
16 |
60 |
|
3B |
Eradication therapy + L. reuteri DSMZ17648 |
10
56 |
18 |
77.8 |
Table 2 Therapy regimens effectiveness for H. pylori infection
So, the prolonged L. reuteri DSMZ17648 probiotic course for 56days was more effective both with monotherapy (60%) and with adjuvant therapy additional to standard eradication therapy (77.8%). Therefore, these subgroups B were further analysed in more detail. Characteristics of patients from subgroups B (1B, 2B, 3B) are presented in Table 3. The groups were comparable in gender and age composition and did not have statistically significant differences.
|
Indicator |
Group 1В |
Group 2В |
Group 3В |
All participants |
Comparison of Group 3В and Group 2В (p) |
Comparison of Group 1В and Group 2В (p) |
|
Qualitative indicators, n (%) |
||||||
|
Number of patients |
20 (37.0) |
16 (29.6) |
18 (33.3) |
54 (100.0) |
- |
- |
|
Gender |
|
|
|
|
|
|
|
Male |
10 (50.0) |
7 (43.8.0) |
9 (50.0) |
26 (48.1) |
0.744 |
0.749 |
|
Female |
10 (50.0) |
9 (56.3) |
9 (50.0) |
28 (51.9) |
||
|
Quantitative indicators, Me (Q1; Q3) |
||||||
|
Age, years |
14.0 (13.0; 16.0) |
15.0 (13.0; 16.0) |
15.0 (13.8; 16.3) |
15.0(13.0; 16.0) |
0.430 |
0.540 |
Table 3 Characteristics of patients in groups 1B, 2B, 3B
As shown in Tables 4, according to Ammonia breath test (ABT) Helic® results in all three groups by day 28, there was a deceleration in exhaled ammonia concentration growth, indicating a decrease in the amount of H. pylori, but after that, in groups 2B and 3B, some increase in the ammonia level was observed by day 56, while in Group 1B patients, by day 56, ABT Helic® indications continued to improve. In the analysis of Rapid urease test RUT Helpil® data, by day 56 in all three groups there was a decrease of H. pylori in both the corpus and the antrum of the stomach. There were no statistically significant differences in the dynamics of this indicator in the groups.
Indicator |
Compared Visits |
Indicator Dynamics, |
Indicator Dynamics Comparison |
|||||
3В vs 1В |
2В vs 1В |
|||||||
Group 2В |
Group 3В |
Group 1В |
The Mann-Whitney Criteria (U) |
Significance level (p) |
The Mann-Whitney Criteria (U) |
Significance level (p) |
||
Ammonia breath test (ABT) Helic, ΔL, mm |
Visit 2 – Visit 1 |
-4.0 (-5.0; -1.0) |
-5.0 (-7.0; -2.0) |
-2.0 (-5.2; - 1.0) |
82.0 |
0.473 |
72.5 |
0.367 |
Visit 3– Visit 1 |
0.0 (-5.0; 3.0) |
-3.0 (-7.0; -0.1) |
-4.0 (-5.0; -2.3) |
49.5 |
0.335 |
38.0 |
0.093 |
|
Rapid urease test (RUT) Helpil: corpus, points |
Visit 3– Visit 1 |
-1.0 (-1.0; 0.0) |
-0.5 (-1.0; 0.0) |
-0.0 (-1.0; 0.0) |
127.5 |
0.529 |
136.0 |
0.399 |
Rapid urease test (RUT) Helpil: antrum, points |
Visit 3 – Visit 1 |
-1.0 (-2.0; -1.0) |
-1.0 (-2.0; 0.0) |
-1.0 (-1.0; 0.0) |
121.5 |
0.413 |
114.5 |
0.127 |
Histology: H. pylori (corpus), points |
Visit 3 – Visit 1 |
-1.0 (-1.0; 0.0) |
0.0 (-1.0; 0.0) |
0.0 (-0.8; 0.0) |
113.0 |
0.248 |
99.5 |
0.032 |
Histology: H. pylori (antrum), points |
Visit 3– Visit 1 |
-1.0 (-2.0; 0.0) |
-1.0 (-1.3; 0.0) |
-1.0 (-1.0; 0.0) |
131.0 |
0.636 |
143.0 |
0.561 |
Inflammatory Index (corpus), points |
Visit 4 – Visit 1 |
-3.0 (-3.4; -2.0) |
-2.0 (-4.0; -0.4) |
-2.0 (-3.0; 0.0) |
125.0 |
0.507 |
102.5 |
0.063 |
Inflammatory Index (antrum), points |
Visit 3– Visit 1 |
-3.5 (-4.0; -1.6) |
-2.0 (-4.0; -1.4) |
-2.3 (-4.0; -1.5) |
119.5 |
0.396 |
135.0 |
0.423 |
Atrophy Index (corpus), points |
Visit 3 – Visit 1 |
-0.5 (-1.0; -0.1) |
-0.3 (-0.5; 0.0) |
0.0 (0.0; 0.0) |
79.5 |
0.019 |
64.5 |
0.001 |
Atrophy Index (antrum), points |
Visit 3 – Visit 1 |
-1.5 (-2.0; -0.5) |
-0.8 (-1.0; 0.0) |
-0.5 (-1.0; 0.4) |
84.0 |
0.039 |
81.5 |
0.011 |
Table 4 Comparative evaluation of the test results dynamics for H. pylori detection and histological examination data for gastric mucosa biopsy specimens in the groups
In the pairwise comparison, that is presented in Table 5, ABT Helic® breath test results showed that by day 28 a statistically significant decrease in ammonia level was noted in all three groups, but by day 56, further reduction was maintained only in groups 3B and 1B, where the children took a probiotic. In the placebo group 2B, the indices did not statistically differ from the baseline. In comparison of the state at the time of the start with the end of treatment, a significant decrease of H. pylori abundance in the antrum was noted in all three groups, and in the corpus of the stomach – in groups 2B and 1B only.
Indicator |
Compared Visits |
Group 2В |
Group 3В |
Group 1В |
|||
Wilcoxon test results (Z) |
Significance Level (p) |
Wilcoxon test results (Z) |
Significance Level (p) |
Wilcoxon test results (Z) |
Significance Level (p) |
||
Ammonia breath test (ABT) Helic, ΔL, mm |
Visit 2 – Visit 1 |
-2.807 |
0.005 |
-3.186 |
0.001 |
-2.470 |
0.014 |
Visit 3 – Visit 1 |
-0.892 |
0.373 |
-2.132 |
0.033 |
-3.065 |
0.002 |
|
Rapid urease test (RUT) Helpil: corpus, points |
Visit 3 – Visit 1 |
-2.496 |
0.013 |
-1.604 |
0.109 |
-2.111 |
0.035 |
Rapid urease test (RUT) Helpil: antrum, points |
Visit 3 – Visit 1 |
-3.244 |
0.001 |
-3.078 |
0.002 |
-2.942 |
0.003 |
Histology: H. pylori (corpus), points |
Visit 3 – Visit 1 |
-2.919 |
0.004 |
-2.310 |
0.021 |
-1.730 |
0.084 |
Histology: H. pylori (antrum), points |
Visit 3 – Visit 1 |
-2.889 |
0.004 |
-2.950 |
0.003 |
-3.556 |
<0.001 |
Inflammatory Index (corpus), points |
Visit 3 – Visit 1 |
-3.305 |
0.001 |
-2.547 |
0.011 |
-2.962 |
0.003 |
Inflammatory Index (antrum), points |
Visit 3 – Visit 1 |
-3.162 |
0.002 |
-3.303 |
0.001 |
-3.277 |
0.001 |
Atrophy Index (corpus), points |
Visit 3 – Visit 1 |
-3.111 |
0.002 |
-1.386 |
0.166 |
-1.890 |
0.059 |
Atrophy Index (antrum), points |
Visit 3 – Visit 1 |
-3.201 |
0.001 |
-2.789 |
0.005 |
-1.830 |
0.067 |
Table 5 Pairwise comparison of test results for H. pylori detection and gastric mucosa biopsy specimens histological examination data in the groups
Thus, both direct (histologically) and indirect (by urease activity and breath test) assessment of gastric mucosa for H. pylori abundance, showed a decrease in all groups (Table 4). According to histology, no statistically significant differences between groups in the antrum of the stomach were found. In the corpus of the stomach in group 1B patients, H. pylori amount in dynamics was reliably and significantly less than the one in group 2B. That is, the L. reuteri DSMZ17648 monotherapy equally or even more effectively reduced the abundance of H. pylori than the standard placebo regimen.
Histological assessment of the gastric mucosa showed that the Inflammatory Index in both parts of the stomach decreased in all three groups, and its dynamics between the groups did not differ statistically. Pairwise comparison between the initial and final indices in each group showed a statistically significant decrease in this index (Table 5) a decrease in the Atrophy Index was also observed in all groups.
From the clinical point of view, dynamics of the disease symptoms is essential. Results of the assessment of the rate of abdominal pain relief in participants of the study are presented in Table 6.
|
Group |
Abdominal pain retention, days |
Comparison of abdominal pain retention periods |
||||||||
|
In Group 3В compared to Group 2В |
In Group 1В compared to Group 2В |
|||||||||
|
Kaplan-Meier method |
Mann-Whitney Criterion (p) |
Kaplan-Meier method |
Mann-Whitney Criterion (p) |
|||||||
|
Me (Q1; Q3) |
95% Conf.int. for Me |
Lagrangian test (p) |
Breslow Criteria (p) |
Tarone-Ware Criterion (p) |
Lagrangian test (p) |
Breslow Criteria (p) |
Tarone-Ware Criterion (p) |
|||
|
Group 2В |
4,0 (3,0; 4,0) |
3,6; 4,4 |
0.068 |
0.059 |
0.062 |
0.059 |
0.127 |
0.145 |
0.135 |
0.178 |
|
Group 3В |
3,0 (3,0; 4,0) |
2,4; 3,6 |
||||||||
|
Group 1В |
3,0 (3,0; 4,0) |
2,5; 3,5 |
||||||||
Table 6 Evaluation of results. Dynamics of abdominal pain relief in the groups
As shown in Table 6, in Groups 3B and 1B patients, the median time during which abdominal pain relief occurred was lower than the one in Group 2B patients. An analysis of the onset time of the effect using both Kaplan-Mayer method and Mann-Whitney test showed differences between group 3B and group 2B as close to statistically significant.
During the observation time, cases of diarrhoea were registered, which was observed only in groups where patients received antibiotics: 3 patients in group 2B and 1 patient in group 3B, while in group 1B there was no diarrhoea in any of the children. Statistical comparison of diarrhoea incidence between Group 2B and 3B showed no statistically significant differences (p=0.323), while the differences between Group 2B and 1B were close to statistically significant (p=0.078).
Standard H. pylori infection therapy includes 2 antibiotics and a proton pump inhibitor (triple therapy), optional adding bismuth preparations increases therapy effectiveness (quadrotherapy).9,27 Despite the use of two antibiotics in standard regimens simultaneously in high doses, and prolongation of treatment up to 14days, eradication therapy results leave much to be desired. This is due to, above all, the increasing antibiotic resistance of H. pylori, particularly to clarithromycin and metronidazole.12
The latest data on the significance of not only H. pylori, but also non-Helicobacter microbiota surrounding it in the progression of pathological process in the stomach,5-8 lead to the search for alternative therapies aimed at correcting microbiome of the stomach as a whole. Probiotics can be a promising and positive direction of therapy, given their ability to safely and complexly affect gastric microbiota composition, protective mucous barrier, and the immune system.28
Previous studies have shown the effectiveness of probiotics in reducing the side effects of standard therapy.29–34 But they could provide new preventive options to patients by lowering the risk for gastric ulcer or carcinoma, thereby avoiding severe adverse effects and treatment expenses. We have conducted a double-blind, randomized, placebo-controlled trial of the therapy efficacy for H. pylori infected children with chronic gastritis by spray-dried L. reuteri DSMZ17648, which were administered both as adjuvant to standard eradication therapy and as monotherapy. In a previous placebo-controlled proof-of-concept in vivo study Lactobacillus reuteri strain DSMZ17648 (Pylopass™ / Lonza) was tested. A significant reduction in 13C-urea breath test (UBT) indicated reduction of bacteria load after active treatment, while no effects were found for placebo control in asymptomatic humans after the two-week supplementation period.18 Lactobacillus reuteri is found in both human breast milk as well as the microbiota of the gastrointestinal tract.35,36 Strains of L. reuteri have been shown to confer health benefits in a variety of cases, including infant colic.37 gastrointestinal disorders in children and feeding intolerance in pre-term infants. Inhibitory effects of L. reuteri (ATCC 55730) on H. pylori have been reported as well.37
Our study has showed that long-term L. reuteri DSMZ17648 probiotic therapy (56days) has the effectiveness almost similar to standard quadrotherapy with two antibiotics and bismuth (respectively 60% and 68.8% H. pylori eradication), and the L. reuteri DSMZ17648 supplemented to traditional therapy increases its efficiency by 9% (from 68.8% to 77.8%). H. pylori eradication rate correlates to probiotic monotherapy duration: 50% after 28days and 60% after 56days of treatment. L. reuteri DSMZ17648 administration allowed to achieve a significant reduction in ammonia level according the ammonia breath test (ABT) results, which observed by day 28 and kept the same level to day 56, while in the placebo group, ammonia concentration, that was achieved by the 28th day, went up again by day 56 reaching values that were not statistically different from the first ones. This reduction in ammonia level may indicate to eradication of H. pylori after probiotic therapy, and possibly also reflects the metabolic activity of gastric microbiome as a whole.
We noted reducing the side effects of standard therapy with antibiotics, particularly diarrhoea, in patients, taking L. reuteri DSMZ17648, and higher rate of abdominal pain relief. Even more important option was significant reduction of gastric mucosa inflammation. This was noted both for monotherapy and adjuvant therapy with L. reuteri DSMZ17648. Even in patients taking L. reuteri DSMZ17648, who did not achieve a complete H. pylori eradication, clinical, endoscopic and histological improvement was recorded. Probiotic treatment is suggested to influence at gastric microbiological composition and favour to restoration of immunological, metabolic and protective functions.
Little has been known about the modification of the gastric microbiota that results from chronic H. pylori infection in children and the interactions with other members of the gastric ecosystem. Our study in children with chronic gastritis has confirmed the earlier data obtained in adults that H. pylori infection changes microbiome of the stomach. We have found that changes in gastric microbiome are as more prominent as longer H.pylori infection and correlate with the severity of inflammation and atrophy. In H. pylori (+) children, and in adults alike, we detected a decrease in gastric microbial diversity and predominance of Proteobacteriae. It is known that H. pylori as well as other members of Proteobacteriae, have a significant pro-inflammatory activity, enhance proliferation and apoptosis.38 Therefore, patients with a high abundance of H. pylori have been found to have a high degree of suppression of other gastric microbiota and more severe mucosal inflammation, in some cases we found initial signs of atrophy. In children with small amount of H. pylori the surrounding microbiota kept diversity because of short history of disease, that was similar to H. pylori (-) children. As a result, in these patients, inflammatory changes of the gastric mucosa were mild. That is, gastric microbiome, as a complex ecosystem, regulates the process of inflammation, regeneration of epithelium and creates opportunities for the development and progression of the disease, or, conversely, could prevents it.39 Probably not the presence of H. pylori itself, but the changes initiated by it, such as the activity of inflammation, the presence of atrophy and intestinal metaplasia, their extent and location, and the duration of the disease are crucial for changing the composition of the gastric microbiome as a whole.
Traditional strategy of eradication therapy, including two antibiotics, can lead to disturbances of the host microecology. So the concept of probiotic therapy, especially monotherapy, is aimed at the long-term and gradual correction of the gastric microbiome. The main outcome of this approach is not only eradication of H. pylori, although, as we have shown, this task is achievable after long-term course of L.reuteri, but rather would have opportunity to stop the progression of chronic gastritis and reach the healing of gastric mucosa. This strategy, in our opinion, is particularly acceptable for children, because it allows reaching complete mucosal healing in the early course of chronic gastritis and provides a favourable long-term prognosis, which is especially important for protection from more severe disease: peptic ulcer and gastric cancer.
It is known that anti-Helicobacter action of probiotics is strain-specific.12 So far, coaggregation effect on H. pylori has been proved only for L. reuteri DSMZ17648,15 and only this strain showed such a high H. pylori eradication rate in monotherapy. Certainly, our study is too small to speak convincingly about the solution of the important problem of H. pylori-associated gastritis treatment in children. Our results of H. pylori eradication with L. reuteri DSMZ17648 monotherapy are insufficient in comparison with those recommended for eradication regimens.25. However, if we weigh the benefit and risk of traditional and probiotic therapy, considering the safety of probiotic therapy and its positive impact at the gastric microbiome, probiotics look much more useful than the continuous increase in dose and duration of antibiotic therapy. We believe that, in children with chronic H. pylori-associated gastritis, L. reuteri DSMZ17648 monotherapy has advantages over standard triple therapy, as it better cures clinical manifestation and gastric mucosa inflammation, which is most likely due to the correction of the state of gastric microbiota as a whole.
Further studies may be aimed at finding more effective strains and microbial metabolites, evaluating longer and more regular intake of probiotic products, which ultimately can fundamentally change the concept of anti-Helicobacter therapy and reduce the risk of developing more severe forms of H. pylori-associated disorders.
The authors thank Svetlana A. Fadina and Anna N. Krupina for providing the patients with corresponding schedule treatment and Maria Suvorova for technical support of the study.
Conceptualization, Elena A.Kornienko; Methodology, Elena A.Kornienko; Software, Sergey V. Ivanov ; Validation, Natalia I. Parolova; Formal Analysis, Yulia D. Kondratenko; Investigation, Natalia I. Parolova, Dmitry A. Polev ; Resources, Pavel A. Zykin; Data Curation, Mikhail M. Zakharchenko; Writing – Original Draft Preparation, Natalia I. Parolova, Dmitry A. Polev ; Writing – Review & Editing, Elena A.Kornienko; Supervision, Elena A.Kornienko.
The authors declare no conflict of interest.
None.

©2020 Kornienko, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.